Abstract
Objective: To report a case of rectal perforation in a patient receiving bevacizumab for glioblastoma multiforme (GBM).
Case Summary: A 63-year-old female with GBM who had experienced adverse reactions to temozolomide was treated with bevacizumab (10 mg/kg every other week). After five cycles of chemotherapy, the patient had fever and severe abdominal pain for 3 days. Abdominal computed tomography showed pneumoperitoneum and multiple diverticula in the sigmoid colon. Under the impression of hallow organ perforation, the patient underwent an emergency operation. The patient was discharged 8 days after the surgery and received only supportive care and nutritional supplements for GBM.
Discussion: Gastrointestinal perforation is a rare but a potentially lethal complication of bevacizumab treatment; the risk varies with tumor types. There are few case reports of rectal perforation associated with bevacizumab, particularly of patients with GBM. This case was classified as a possible adverse reaction of bevacizumab according to the Naranjo probability scale.
Conclusions: Although the occurrence of this side effect is rare and the pathophysiology is not yet understood, clinicians should be alert for signs and symptoms of gastrointestinal tract disorders in patients undergoing therapy with bevacizumab.
Key words: bevacizumab, glioblastoma multiforme, rectal perforation
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary malignant brain tumor in humans. The standard therapy is maximum surgical resection consistent with preserving neurologic function. Radiotherapy plus the alkylating agent temozolomide has been shown to improve progression-free and overall survival after surgery.1 GBM is a highly vascular tumor; majority of patients relapse following initial therapy. An alternative therapeutic approach that inhibits angiogenesis is expected to prevent recurrent GBM.
Vascular endothelial growth factor (VEGF) plays an important role in the development of the abnormal vasculature observed in malignant gliomas. Bevacizumab (Avastin), a monoclonal antibody that inhibits angiogenesis by targeting VEGF, was approved by the U.S. Food and Drug Administration in 2009 for recurrent, progressive disease following standard GBM therapy. Bevacizumab, alone or in combination with irinotecan, carboplatin, erlotinib, or etoposide, is effective and well tolerated in GBM treatment.2 The major side effects of bevacizumab include hypertension, venous thromboembolism, proteinuria, and wound-healing complications. Hemorrhages, such as intracellular hemorrhage, gingival bleeding, conjunctival hemorrhage, and hematuria have also been reported. The incidence of gastrointestinal perforation ranges from 0.3-2.4 %.3 Though rare, this adverse reaction is potentially fatal. We present a case of rectal perforation that occurred in a patient who had received bevacizumab as a treatment for GBM.
Case Report
A 63-year-old woman had a history of hypertension, which was under control. Her regular medications included lisinopril and verapamil, which she had been taking for at least 7 years without any adverse effects. The acute onset of slurred speech and left-side weakness with ataxia was noted in July 2012. The patient was diagnosed with GBM and underwent frontotemporal craniotomy for tumor excision on August 3, 2012. Oral temozolomide 120 mg daily and radiotherapy were administered after the surgery, but they led to severe nausea and vomiting. Because of temozolomide intolerance, the patient refused to take this medication. Thus, six doses of bevacizumab (10 mg/kg every other week) were administered from March 22, 2013.
After the fifth doses of chemotherapy, the patient had fever and severe abdominal pain for 3 days. A physical examination revealed fever (38.2℃), tachycardia (123 bpm), and hypertension (165/102 mmHg). Abdominal examination was consistent with tenderness, rebounding pain, muscle guarding, and hypoactive bowel sound. Laboratory tests revealed normal serum amylase and lipase, as well as slightly elevated GOT (51 U/L) and GPT (58 U/L) (reference range 0-40 U/L). An abdominal computed tomography scan showed pneumoperitoneum and multiple diverticula in the sigmoid colon (Figure 1). Upon diagnosis of hollow organ perforation, the patient underwent an emergency operation (Hartmann operation and peritoneal toilet) on May 25, 2013. After emergent surgical intervention, the patient was referred to intensive care unit (ICU) for intensive care. In ICU, intraoperative findings revealed upper rectal perforation and sigmoid colon diverticulitis with intra-abdominal abscess, empirical antibiotics as Vancomycin 1 g QD and doripenem hydrate 0.5g Q8H was given for intra-abdominal infection. After improved condition, nasogastric tube (NG) diet was tried and the patient had good digestion. Otherwise, the abdomen wound had good healing and mild discharge. Thus, the patient was discharged 8 days after the surgery. After evaluation, bevacizumab associated rectum perforation was suspected, and the patient received only supportive care for GBM. The patient died on October 3, 2013 due to respiratory failure.
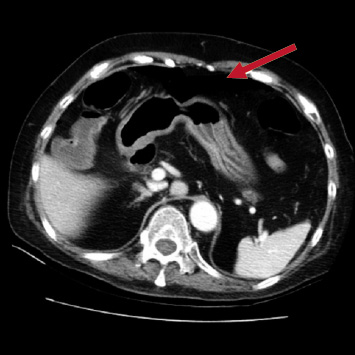
Figure 1. Abdominal computed tomography showed pneumoperitoneum at the sigmoid colon
Discussion
Bevacizumab, a recombinant humanized monoclonal antibody against VEGF, has been shown to treat several types of cancer. It is a first-line therapy for metastatic breast cancer, and it is used to treat metastatic carcinoma of the colon or rectum, as well as glioblastoma that recurs after previous treatment.4 In a prospective phase II study, forty eight patients were administered bevacizumab (10 mg/kg) every 2 weeks on a 4-week cycle for recurrent GBM treatment. The primary endpoint of the study was progression-free survival (PFS). Tumor responses obtained via an MRI were also assessed using Macdonald and Levin criteria. The median PFS was 16 weeks [95% confidence interval (CI), 12-26 weeks], and the PFS at 6 months was 29% (95% CI, 18%-48%). The overall response rate based on Macdonald criteria was 35% and on Levin criteria was 71%.5 In summary, the administering bevacizumab as a single agent has significant biologic and antiglioma activity in patients with recurrent GBM.
The major adverse events (≧ grade 3, according to the National Cancer Institute Common Terminology Criteria for Adverse Events) associated with bevacizumab therapy are hypertension (4.2-16.0%), venous thromboembolism (2.0-12.6%), proteinuria (approximately 3.2%), and wound-healing complications (approximately 2.4%).3 Gastrointestinal perforation is a rare but a lethal complication. According to a meta-analysis of 17 randomized controlled trials, the incidence of gastrointestinal perforation is 0.9% (95% CI, 0.7-1.2) among patients receiving bevacizumab and the mortality rate is 21.7% (95% CI, 11.5–37.0).6 The risk of gastrointestinal perforation varies with tumor type. It is higher in patients with colorectal carcinoma or renal cell cancer. Bevacizumab-associated perforation seems to be dose-dependent; the relative risk was higher at a dose of 5 mg/kg than at 2.5 mg/kg per week.4 In the case reported here, the dose was 10 mg/kg q2w.
The mechanism of bevacizumab-associated perforation is not well understood. Bevacizumab may limit blood flow to the splanchnic vasculature. The lack of blood flow to the bowel could potentially lead to perforation. Another risk factor suggested is the use of bevacizumab within 28 days of a colorectal operation. The large intestine is the most common site of bevacizumab-associated perforation.7 In the case reported here, we observed perforation and diverticulitis of the sigmoid colon, as well as pneumoperitoneum. Rectal perforation developed after the fifth cycle of bevacizumab therapy, which was approximately the same time as that reported in a previous case series.8 Patients who develop gastrointestinal perforation should permanently avoid the use of bevacizumab. We discussed this case in our ADR committee, with regard to the patient in this report, lisinopril and verapamil were less likely to be the cause of rectal perforation because of their long-term use without adverse reactions. Alternative causes, such as disease induced rectum perforation was excluded by gastroenterologist. According to the Naranjo probability scale9, this case was classified as a probable bevacizumab-related rectal perforation (Table 1).
Table 1. Naranjo adverse drug reaction probability scale of this case
The Naranjo adverse drug reaction probability scale |
Yes |
No |
Do not know |
Score |
1.Are there previous conclusive reports on this reaction? |
+1 |
0 |
0 |
+1 |
2.Did the adverse event occur after the suspected drug was administered? |
+2 |
-1 |
0 |
+2 |
3.Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? |
+1 |
0 |
0 |
+1 |
4.Did the adverse reaction reappear when the drug was readministered? |
+2 |
-1 |
0 |
0 |
5.Are there alternative causes (other than the drug) that could have on their own caused the reaction? |
-1 |
+2 |
0 |
+2 |
6.Did the reaction reappear when a placebo was given? |
-1 |
+1 |
0 |
0 |
7.Was the blood detected in the blood (or other fluids) in concentrations known to be toxic? |
+1 |
0 |
0 |
0 |
8.Was the reaction more severe when the dose was increased or less severe when the dose was decreased? |
+1 |
0 |
0 |
0 |
9.Did the patient have a similar reaction to the same or similar drugs in any previous exposure? |
+1 |
0 |
0 |
0 |
10.Was the adverse event confirmed by any objective evidence? |
+1 |
0 |
0 |
+1 |
Total |
|
|
|
7 |
Conclusions
Although gastrointestinal perforation associated with bevacizumab is rare and the pathophysiology is not yet understood, clinicians should be alert for signs and symptoms of abdominal pain, nasuea, and vomiting in patients undergoing therapy with bevacizumab. If gastrointestinal perforation is diagnosed, bevacizumab administration should be ceased immediately and an appropriate intervention, such as surgery, should be undertaken.
摘要
疑似 Bevacizumab 引起腸穿孔之案例報告
台南市立醫院藥劑科藥師 吳佩霖、黃秋谷
目的:提出一名因治療多型性神經膠母細胞瘤,使用bevacizumab而引起腸穿孔副作用的案例報告
個案簡述:一名患有多型性神經膠母細胞瘤的63歲女性病患,因無法耐受 temozolomide 副作用而改用每兩周靜脈注射 bevacizumab 10 mg/kg,在經過第五個循環的化學治療後,病患發生嚴重的腹痛、發燒至急診就醫,腹部斷層掃描顯示在乙狀結腸處有腹腔積氣與憩室,診斷為腸穿孔,因此緊急接受手術修補;病患在手術後8天情況穩定出院,但之後僅以營養支持等保守性療法治療多型性神經膠母細胞瘤。
討論:胃腸穿孔是 bevacizumab 罕見但具致命性的副作用,發生的風險性於不同癌症有所差異,在大腸直腸癌患者機率最高;在國內外的文獻中,多型性神經膠母細胞瘤患者發生此藥物不良反應僅有少數的個案報告。經 Naranjo 計分表評估後,判斷此病患極可能為 bevacizumab 引起腸穿孔的藥物不良反應。
結論:雖然 bevacizumab 引起腸穿孔的機率為罕見、且病理機制尚不明確,但在使用 bevacizumab 期間,醫療人員仍須密切注意病患是否有腹痛、腹脹等胃腸道方面的不適症狀,並及早採取適當的醫療處置。
References:
1. Ahmed R, Oborski M, Hwang M, Liberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res 2014;6 149-70.
2. Friedman HS, Prados MD, Wen PY,et al: Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40.
3. Narita Y. Drug Review: Safety and efficacy of bevacizumab for glioblastoma and other brain tumors. Jpn J Clin Oncol 2013;43:587-95.
4. Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009;10:559-68.
5. Kreisl TN, Kim L, Moore K, et al: Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2008; 27:740-5.
6. Moen MD. Bevacizumab: in previously treated glioblastoma. Drugs 2010;70:181-9.
7. Scappaticci FA, Fehrenbacher L, Cartwright T, et al: Surgical wound healing complications in metastaticcolorectal cancer patients treated with bevacizumab. J Surg Oncol 2005;91:173-80.
8. Badgwell BD, Camp ER, Feig B,et al: Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 2008;19:577-82.
9. Naranjo CA, Busto U, Sellers EM, et al: A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.

