Abstract
The 2009 swine flu pandemic is caused by a novel influenza A (H1N1) virus. Phylogenetic study reveals that this virus possesses genes derived from viruses of swine, avian and human origins through several reassortments. This virus is capable of spreading from person to person readily resulting in a worldwide pandemic flu as of June 11, 2009. The flu pandemic timeline for the past century demonstrates that all the causative viruses for the pandemics as well as the seasonal epidemics are attributed to the type A influenza. The novel H1N1 virus has established itself rapidly as a dominant influenza A strain in most parts of the world. So far, most, if not all, of the pandemic patients suffers a mild illness. The majority (90%) of the world's population lives in the northern hemisphere who is as yet to experience the first fall and winter seasons, when it is most prone for flu outbreaks due to the cold and dry weather. A second wave of the pandemic attacks seems inevitable.
Key words:
Swine flu, novel influenza A (H1N1) virus, 2009 flu pandemic, gene reassortment, seasonal flu epidemic, flu pandemic timeline
Initial outbreaks
Towards mid-April this year (2009) the swine flu that broke out in Mexico causing numerous cases of infection and claiming many lives has caught the international health authorities by surprise. Why not the H5N1 avian flu or the SARS-like epidemic? The whole world has been geared to face any challenges from the two for many years, since the major outbreaks of the H5N1 avian flu with human casualties as of late December 2003 and ongoing to date. It is noted that after H5N1 emerged widely in Asia in 2003, killing about 60-80 percent of the humans infected by the virus, many countries took steps to prevent a similar crisis. SARS was rampant for approximately six months between February and July 2003 with a case fatality rate or CFR of approximately 10%. It even spread from Asia to the North America in a cluster. The current swine flu epidemic spread quickly across the borders to the immediate neighbor, the U.S.A. and even further north to Canada. The panic was understandably reflected by the sharp world wide plummeting of the stock market during one week in late April. The rest of the world has not been left unscathed. As the time goes by the H1N1 virus has invaded other continents beyond the North America, such as South America, Europe, Asia, Australia and Africa. The number of countries affected has increased rapidly. By July 21, the WHO (World Health Organization) reported 143,841 laboratory-confirmed cases and 813 deaths (CFR at 0.6 %) worldwide from 137 affected countries. A month prior, all 50 states in the United States, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands had reported novel H1N1 infection.
WHO declares: 2009 Flu pandemic
On June 11th, the WHO announced that its alert level for H1N1 influenza should be raised to phase 6 - see the following chart. A global pandemic is officially under way. This is just to signal the beginning of the so-called first summer wave of the pandemic (see Fig. 1. Pandemic Influenza Phases ).
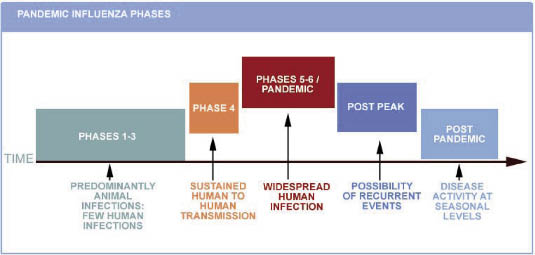
Fig. 1. Pandemic Influenza Phases
Phase 6 criteria: In addition to the criteria defined in Phase 5, the same virus has caused sustained community-level outbreaks in at least one other country in another WHO region1 (Courtesy of the WHO).
The WHO has subsequently admitted on July 16 that the pandemic is unstoppable. As of this date, the WHO will give up all reports on lab-confirmed total cases, whilst fatal cases due to the novel H1N1 should be lab-confirmed. The argument is that it is either wasteful or pointless to carry out costly diagnosis, as the new flu has become so rampant in most, if not all, communities and countries. Because it is a mild flu, most people have chosen to stay home until self-recovery. So, the reporting of totals has become much too inaccurate and actually meaningless. In mid-July, experts believe that in the USA alone there might have been more than a million cases. However, the WHO and the CDC (Centers for Disease Control and Prevention, USA) stress on the significance of reports for unusual clusters of the new H1N1 virus and the related severe cases by the national health authorities. In particular, any drug-resistant cases should be reported and the strains involved should be shared among the international flu scientists. As from now both the WHO and the CDC consider that to estimate the spread of the “unstoppable” virus through computer-modeling is more sensible than counting the total cases.1,2
Clinical symptoms
A recent study carried out by the CDC3 has shown that sufferers of the new H1N1 tend to come down with fever (93%) and cough (83%). Besides the two most common symptoms, the following signs are also seen: as shortness of breath (54%), fatigue/weakness (40%), chills (37%), myalgias (36%), rhinorrhea or running nose (36%), sore throat (31%), headache (31%), vomiting (29%), wheezing (24%), diarrhea (24%). The manifestations and the mode of transmission are very similar to those of the seasonal influenza. Patients with at least two signs of the acute respiratory illness should call their care providers promptly. The duration of illness is typically 4-6 days. The infectious period for a confirmed case is defined as 1 day prior to the onset of symptoms to 7 days after onset. The CDC has noted that most infections continue to be mild and recovery is extremely quick.
Viral origins
Phylogenetic analysis through international collaboration has produced insights in the origins of the novel 2009 flu. Obviously it is a human flu in that it is spreading from person to person. But unambiguously this virus has its immediate origins in pigs. Specifically it is the product of reassortment events between at least two swine flu variants. Moreover, one of the variants has some genes linked to an avian virus and human H3N2 virus (one of the circulating seasonal flu agents to date). In short, the novel 2009 swine flu virus is an influenza type-A virus consisting of genes derived from viruses of swine, avian and human origins through several reassortments4,5. Evidence from multiple outbreak sites demonstrates that the H1N1 pandemic 2009 virus has rapidly established itself and is now the dominant influenza strain in most parts of the world. The pandemic will persist in the coming months as the virus continues to move through susceptible populations.
Seasonal flu epidemics
Influenza epidemics recur annually mostly in the cold dry winter seasons. Annual influenza epidemics are estimated to affect 5-15% of the global population. Most cases are mild, but may cause severe illness in 3-5 million people and around 250,000-500,000 deaths worldwide. In industrialized countries severe illness and deaths occur mainly in the high-risk populations of infants, the elderly, and chronically ill patients.6-8
Pandemic timeline
In the past century, Influenza A virus strains caused three major global epidemics: the Spanish flu in 1918 (caused by influenza A virus subtype H1N1), Asian flu in 1957(caused by influenza A virus subtype H2N2) and Hong Kong flu in 1968-69 (caused by influenza A virus subtype H3N2). These pandemics were all caused by various subtypes of Influenza A virus that had undergone major genetic reassortments and for which the population did not possess significant immunity. The overall effects of these pandemics and epidemics are summarized in the table below.7,8
It is estimated that anywhere from 20 to 100 million people were killed worldwide. An estimated 500 million people, one third of the world's population (approximately 1.6 billion at the time), became infected. The estimated numbers are definitely very imprecise given the fact that the influenza virus was only formally discovered in 1933 by Smith and colleagues.9 By the same token, since no laboratory techniques were available at that time for confirmation other than clinical manifestations, the gross estimated figures include both the pandemic and the seasonal flu sufferers. It was no doubt that a dreadful pandemic took place globally that killed more people than those (16 million, 1% of the 1.6 billion of the world's population) killed in the World War I including both military personnel and civilians (Table 1.).
Table. 1. 20th century flu pandemics
Pandemic |
Year |
Influenza A virus |
People infected |
Deaths |
Case fatality rate (CFR) |
1918 flu pandemic |
1918-19 |
H1N1 |
0.5 to 1 billion (near 50%) |
20 to 100 million |
>2.5% |
Asian flu |
1956-58 |
H2N2 |
2 million |
<0.1% |
|
Hong Kong flu |
1968-69 |
H3N2 |
1 million |
<0.1% |
|
Seasonal flu* |
Every year |
mainly A/H3N2, A/H1N1, and B |
5–15% (340 million - 1 billion) |
250,000-500,000 per year |
<0.05% |
* Seasonal flu is not a pandemic, but is listed to compare the several flu strains endemic in humans which produce seasonal flu with the rare new strain that results in a flu pandemic.8
Comparison: 1918 and 2009 pandemics
Notwithstanding the fact that there are substantial differences between the living environments of now and 90 years ago, in terms of demography, public health system, medications, technologies, transportations, scientific knowledge and others, comparative studies can be carried out for predicting the outcomes of the current pandemic. Although the two pandemics are both caused by H1N1 viruses, the two causative influenza type A variants do not share exact biology/virulence. There might be subtle and yet different genetic makeup between the two flu viruses. So far we have not got enough information as to the molecular pathogenesis and the epidemiology of the 2009 novel H1N1 flu. The majority of the world's population resides in the northern hemisphere and has not yet experienced the flu-prone winter season since the advent of the novel swine flu in April in Mexico. It remains to be seen whether this flu will be as powerful/devastating as the 1918 flu. Historical and epidemiological data are inadequate to identify the geographic origin of the 1918 pandemic flu. Most of its victims were healthy young adults aged between 25 and 34 (see Fig. 2 for the solid curve), in contrast to most seasonal influenza outbreaks which predominantly affect juvenile, elderly, or otherwise weakened patients (see Fig. 2 for the dotted curve).
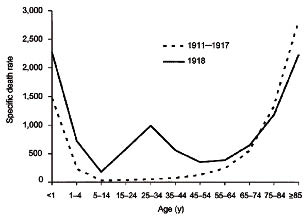
Fig. 2. Death-age group relationship - the 1918 flu pandemic versus seasonal flu epidemics.
CDC studied the hospital records of 268 patients hospitalized with novel H1N1 flu early on during the outbreak. The number of deaths was highest among people 25 to 49 years of age (41%), followed by people 50 to 64 year of age (24%) and people 5 to 24 year of age (16%) (Fig. 3). This is a very different pattern from what is seen in seasonal influenza, where an estimated 90% of influenza-related deaths occur in people 65 years of age and older (see Fig. 2).
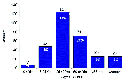
Fig. 3. Novel H1N1 U.S. Deaths, By Age Group3
In Fig. 3, the pattern appears like an “A” shape rather than a “W” as seen in the 1918 pandemic (see Fig. 2 for the solid curve). However, the two letters share a common middle protruding point in that all points to the vulnerability of the 25-49 age group. The 0-4 and the ≧65 age groups as well as the people with chronic diseases do not appear to be stricken badly at present. When the pandemic advances to its maturity the “A” shape may become subject to transformation with significant statistical data due to be collected globally. In the seasonal flu epidemics, a typical “U” curve is observed with the 0-4 and the ≧65 age groups sticking out at the both ends of the U letter (see Fig. 2 for the dotted curve).
In Fig. 2, the 25-34 age group stands out as a single peak in the middle of the W-shaped curve for the pandemic flu, whilst the same age group remains flat in the annual U-shaped curve observed for the seasonal flu epidemics prior to 1918. Why the 1918 H1N1 virus had its surprising effect on younger and healthier people, particularly those aged between 25 and 34. Kobasa and co-workers reported that monkeys (Macaca fascicularis) infected with the recreated 1918 pandemic strain exhibited classic symptoms of the pandemic, an overreaction of the immune system, cytokine storm.10
1918 pandemic in Taiwan
The Spanish flu occurred in Taiwan three months later than the United States / Europe, and coincided with most of other Asian countries. There were three waves of the 1918 flu pandemic recurrences in Taiwan: wave I (June to mid-September, 1918), wave II (late October to the end of December, 1918), and wave III (December, 1919 to February, 1920). Within wave I, although more than usual numbers of people got flu-like symptoms in the hot summer days, little official record by the local health authorities could be discovered retrospectively. A dramatic surge of cases erupted in the second wave. In this period, it caused more than 779,532 cases of infection (413,984 males and 365,539 females) and 25,394 deaths (CFR at 3.3%). The wave II subsided after the end of December of 1918, a relatively calm period took over for nearly nine months until mid October of 1919 when cases of flu started to rise. The pandemic flu resumed as of December and lasted until the end of February 1920 and then began to die out. In this wave, another 148,885 people were infected, and among them19,244 people succumbed (CFR at 12.9%). The high CFR could be due to sudden emergence of a mutated H1N1 with augmented virulence. By the same token, the abrupt disappearance of the pandemic flu could likely be attributed to a weakened virus and possession of immunity by the general population. In total, the pandemic flu infected 928,408 people and caused 44,638 deaths (CFR at 4.8%).
It is unambiguous to note that the geographic origin of the pandemic flu in Taiwan was Japan where 257,363 deaths were attributed to influenza by July 1919, giving an estimated 0.425% mortality rate (or 524 per 100,000 people). The initial entry point was the Keelung harbor. The feverish Japanese soldiers of two shiploads disembarked at this international seaport shortly before June 1918 and the pandemic flu started to spread in the military battalions, then the Japanese civilians and finally the vast Taiwanese population.11
The total population of Taiwan was 3,669,687 at that time according to the census kept prior to the pandemic. This means that the infection rate of the pandemic against the population stands at approximately 25.3%, the gross mortality rate at 1.2 % (or 1,200 per 100,000 people). It was noted that a “U” curve instead of a “W” curve was observed in the mortality profile for the 1918 pandemic in Taiwan (data not shown). A plausible explanation for this is that throughout the flu pandemic period of two years (1918 June-1920 June), Taiwan enjoyed a rather peaceful and stable social status. Inversely, in March of 1918, most of the West European cities were quite devastated and war-torn after four years of World War I. The exhausted young soldiers were crowded in military camps and in trains by hundreds or thousands to be sent home when the pandemic flu erupted. Although they were not killed by a bullet earlier in the battle field, many of them didn't survive the attack by the virus. The official tally of the death toll of the 1918 pandemic is about 21.6 million, the most conservative number12 and that of World War I is 16 million.
Medications
The H1N1 influenza A (swine flu) virus is susceptible to the antiviral drugs oseltamivir [Tamiflu] and zanamivir [Relenza] which are neuraminidase [NA] inhibitors, and the CDC has issued interim guidance for the use of these drugs to treat and prevent infection with swine influenza viruses3. There are other antiviral agents, such as amantadine, rimantadine which are M2 Protein inhibitors. M2 protein (an ion channel), which is required for the viral particle to become "uncoated" once taken inside a cell by endocytosis. Amantadine was approved by the Food and Drug Administration (FDA) on October 1966 as a prophylactic agent against Asian influenza and eventually received approval for the treatment of Influenzavirus A. However they are not recommended because of the drug resistance problem to many influenza strains (e.g. H5N1, H3N2) documented over the past several years.
Suggested regimes of Tamiflu and Relenza for both adult dose and pediatric dose can be found in.13 The more we use the antivirals against the flu, the more we find the drug resistant viruses. A score of Tamiflu resistant H1N1 viruses have been reported due to point mutations at the NA gene. Fortunately, so far, all the Tamiflu-resistant viruses have remained sensitive to Relenza. On the other hand, cases of Relenza resistant H1N1 have been isolated in Australia.14 Australian researchers have reported the first evidence of resistance to Relenza in seasonal influenza A (H1N1) strains.
In 1918 the salts of quinine and aspirin have been most generally used during the pandemic. Nowadays, in addition to the antivirals as mentioned, we are able to apply other basic supportive medications (i.e., hydration, analgesics, cough suppressants) for relieving symptoms. Treatment of hospitalized patients and patients at higher risk for influenza complications should be prioritized. Face masks for various purposes are available, such as healthcare professionals and patients and people working at customs and so on.
The usual vaccine for seasonal influenza administered prior to the flu season is not effective for this viral strain. Effective vaccines against the 2009 novel H1N1 virus are being made by some major drug manufacturers. Whether an effective vaccine will be available in time for preventing the pandemic will be a real challenge.
Prospect of 2009 H1N1 pandemic
The majority of the world's population -about 90% lives in the northern hemisphere while the remaining 10% lives in the southern hemisphere. As northern hemisphere residents, we are about to enter the fall season as of September, when all students will return to school and crowd the classrooms and vacationers come back to their offices. The autumn is the season when the air in the atmosphere begins to get drier and the temperature gets lower than the summer. The mucosal surface of our upper respiratory tracks will be rendered with micro-cracks through which opportunistic microorganisms may enter our body readily to cause infections. As the season advances into the dry and cold winter, the above phenomenon becomes more serious. Also, viral particles in aerosols will remain active longer in the cold weather than in summer. All of these will provide the ideal opportunities for the H1N1 to spread rapidly in the fall and winter seasons.
So far the clinical picture of pandemic influenza is largely consistent across all countries. The overwhelming majority of patients continue to experience mild illness. However, increased risks for some vulnerable groups are well documented, such as women during pregnancy and people with predisposing conditions (asthma, diabetes, immunosuppression, and obesity).15 Studies have detected no signs that the virus has mutated to a more virulent or lethal form. But there is no absolute guarantee that the novel H1N1 will remain dormant without abrupt genetic mutation throughout the coming fall and winter seasons in the northern hemisphere.
References:
1. World Health Organization. Influenza-like illness in the United States and Mexico. WHO Epidemic and Pandemic Alert and Response. Available at <http://www.who.int/csr/don/2009_04_24/en/index.html>. Accessed April 27, 2009.
2. National Center for Biotechnology Information. Influenza Virus Resource. <http://www.ncbi.nlm.nih.gov/genomes/FLU/SwineFlu.html>. Accessed May 4, 2009.
3. CDC USA. Novel H1N1 flu: facts and figures. <http://www.cdc.gov/h1n1flu/surveillanceqa.htm>
4. Swine Flu Might Have Come From Asia. New York Times, June 23, 2009 <http:www.nytimes.com/2009/06/24/health/24flu.html/?hp>
5. Phylogenetic analysis and reassortment history <http://tree.bio.ed.ac.uk/groups/influenza/wiki/aea97/Phylogenetic_analysis_and_reassortment.html>
6. Influenza: Fact sheet. World Health Organization. March 2003. <http://www.who.int/mediacentre/factsheets/2003/fs211/en/>.
7. 1918 flu pandemic. Wikipedia. <http://en.wikipedia.org/wiki/1918_flu_pandemic>
8. Flu Pandemic, an overview by Wikipedia, http://en.wikipedia.org/wiki/2009_flu_pandemic#Historical_context
9. Smith W, Andrewes CH, Laidlaw PP. A virus obtained from influenza patients. Lancet 1933; 222: 66-68.
10. Kobasa, Darwyn; et al. (2007). "Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus". Nature 445: 319-323. doi:10.1038/nature05495.
11. Epidemic Influenza- A survey, p.228 &229, Edwin O. Jordan, 1927
12. 西班牙型流行性感冒. Wikipedia. <http://zh.wikipedia.org/wiki/%E8%A5%BF%
E7%8F%AD%E7%89%99%E5%9E%8B%E6%B5%81%E8%A1%8C%E6%80%A7%E6%84%9F%
E5%86%92?variant=zh-tw >
13. H1N1 Influenza (Swine Flu) [eMedicine/Medscape] http://emedicine.medscape.com/article/1673658-overview
14. Jared Reed. First evidence of Relenza-resistant flu. http://www.6minutes.com.au/articles/z1/view.asp?id=492811
15. Influenza pandemic (H1N1) 2009 (37): second wave plan, WHO. ProMED Digest V2009 #407.
摘要
2009年豬流感全球人類大流行係由一種新種流感A (H1N1)病毒所引起。以基因種源分析得知,本病毒具有豬、鳥、人流感病毒基因歷經多次基因互換重組而成。本病毒能在人類相互間快速傳播終成全球大流行。二十世紀中有過三次全球大流感和每年冬季例行之流感病毒皆屬於A型流感病毒。2009新種流感A (H1N1)病毒已經變成全球主流病毒,本次全球人類大流感受害者之病症普遍地輕微。秋冬是流感盛行季節,全球90%之人口居住在北半球很可能將在今年秋冬遭遇到全球大流感之第二波攻擊。
關鍵字:
豬流感、新流感A (H1N1)病毒、2009全球大流感、基因重整組、季節性流感、全球大流感時程表
作者
美國紐約州依色佳,康乃爾大學生技中心,生化、分子及細胞生物系教授 楊哲安
Robert C. A. Yang, PhD
Consultant
Biobank-Taiwan
D.O.H. Taiwan
Professor
Section of Biochemistry, Molecular & Cell Biology, Biotech Center
Cornell University
Ithaca, N. Y., USA

