Abstract
Leflunomide, a new class of disease-modifying antirheumatic drug (DMARD), used in the treatment of active rheumatoid arthritis (RA). It is a prodrug that is rapidly metabolized to an active metabolite, A771726 with a long elimination half-life; therefore, serious adverse events may occur even after leflunomide treatment has been stopped. In this case report, leflunomide was prescribed to a 53-years-old male patient with history of RA. Unfortunately, forty-five days later he developed a progressive jaundice with elevated liver enzymes. Then acute hepatic failure with liver damage was diagnosed in the following weeks. Leflunomide has commonly been associated with hepatic toxicity. This case was reporting the acute hepatic failure associated with leflunomide, therefore, we strongly recommend health care physician to routinely monitor patient's liver function for minimizing the potential hepatotoxicity with leflunomide therapy.
Key words: hepatic failure, hepatic toxicity, rheumatoid arthritis, disease-modifying antirheumatic drug
Introduction
Leflunomide is a new class of disease-modifying antirheumatic drug (DMARD). Food and Drug Administration (FDA) approved the indication for rheumatoid arthritis (RA) in October 19981. Leflunomide is a prodrug which is converted to an active metabolite A77 1726 by first-pass metabolism in the liver and gastrointestinal tract. A77 1726 is highly plasma protein binding (99.5%), predominantly to albumin2. To have the immunomodulatory and antiproliferative activity in RA, the mechanism of leflunomide is reducing pyrimidine synthesis by inhibiting the dihydro-orotate dehydrogenase3. Because of the leflunomide's prolonged half-life approximate to 15 days in human and the desirability of rapidly attaining a steady state blood level, FDA approved an oral loading dose of 100 mg daily for 3 days, followed by 10 to 20 mg daily1-2. Common adverse events with leflunomide are diarrhea, respiratory infections, nausea, headache, and rash. Elevated liver function tests have been reported4. In March 2001, the European Agency for the Evaluation of Medicinal Products (EMEA) published a warning about the potentially serious hepatic toxicity with leflunomide, and has been highlightened concern over the potential serious hepatic damage5. This case report was reporting leflunomide induced acute hepatic failure. In order to avoid the hepatic damage caused by leflunomide, would like to remind healthy care providers to notice the changing in liver function while patient is using leflunomde.
Case Report
A 53-year-old man has a history of RA and End-Stage Renal Disease (ESRD) with regular hemodialysis. He was admitted to ER due to his conscious was suddenly changed on the way to hospital for hemodialysis. According to the patient's wife described, he felt mild chills and general malaise, but denied fever. On the examination, the patient's heart rhythm was converted, pulse rate was 48, respiratory rate was 20, and blood pressure was 123/87 mmHg. His serum potassium level was 8 mEq/L and then patient was transferred to the intensive care units.
This patient has a medical history of severe RA. His previous RA treatment included hydroxychloroquine and sulfasalazine. Since Dec 2008, his currently maintained treatment was switched to leflunomide 20 mg/day. Liver function tests were checked with normal range at that time. Forty-five days after starting with leflunomide therapy, the progressive jaundice with elevated liver enzyme was noticed and leflunomide was withdrawn. Autoimmune markers were checked as well; antinuclear antibodies (ANA) was homogeneous (1:640X); anti-smooth muscle antibodies (ASMA) was positive (1: 20X) and virus hepatitis markers showed negative.
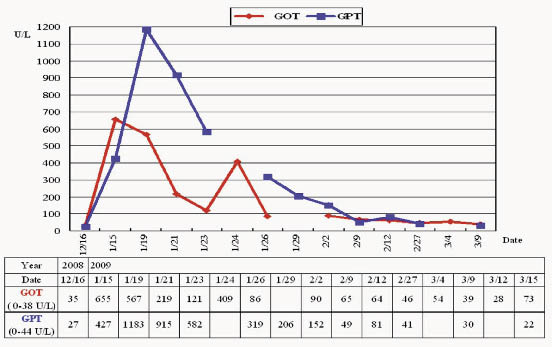
Based on these changes of liver enzymes, the patient was diagnosed with acute hepatic failure. Plasma exchange was performed on 12th day after leflunomide discontinued, but hepatic failure still progressed. Unfortunately, he was critical discharged from the hospital. Figure1 showed the progress of this patient's laboratory data of liver enzymes.
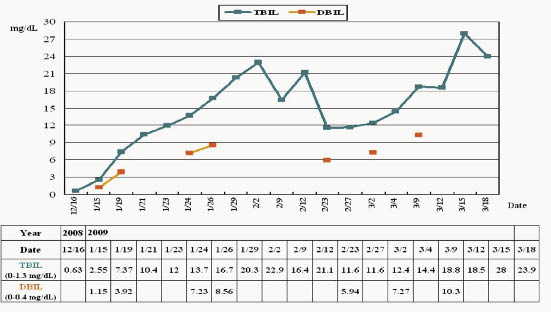
Figure 1. The progress of this patient's laboratory data of liver enzymes. The duration of using leflunomide was from 2008/12/10 to 2009/01/24. GOT= glutamic oxaloacetic transaminase; GPT = glutamic pyruvic transaminaseand; TBIL= total bilirubin; DBIL= direct bilirubin
Discussion
This case illustrated the development of acute hepatic failure as a likely adverse event of leflunomide therapy. The EMEA has raised concerns about the safety profile of leflunomide, especially regarding to hepatotoxicity, pancytopenia and serious skin reactions. In 2002, according to the EMEA finding of 296 hepatic reactions (in 104,000 patient-years of leflunomide exposure), with 129 cases considered serious, there has been heightened patient concern. Also these adverse events with leflunomide are more common in the first 6 months treatment6. However, all the cases in this finding were complicated by associated factors, such as comorbidity (heart failure, alcohol hepatic, pulmonary failure, viral infection, sepsis) and other possibly hepatotoxic comedication.
The majority of adverse drug-induced hepatic events is unpredictable and is either immune-mediated hypersensitivity reactions or is idiosyncratic. The pathogenesis usually involves the participation of a toxic drug or its metabolite9. Variable clinical presentations are ranging from asymptomatic mild hepatic biochemical abnormalities to an acute failure with jaundice, resembling viral hepatitis10.
Because leflunomide has an active metabolite with a long eliminated half-life, monitoring live function are recommended. Serum values of GOT and GPT must be checked before initiated treatment and then monthly during the first 6 months treatment, and every 8 weeks thereafter7. If GPT elevated up to 2- and 3- folds of the upper limit of normal (ULN) range, serious adverse reactions may occur even after leflunomide treatment has been stopped. The Naranjo scale assessment of the possibility of ADR of leflunomide induced acute hepatic failure was“Probable”in this case (Table 1).
Table 1. Naranjo scale assessment of the possibility of ADR
To assess the adverse drug reaction, please answer the following questionnaire and give the pertinent score. |
|||||
Yes |
No |
Do Not Know |
Score |
||
1. |
Are there previous conclusive reports on this reaction? |
+1 |
0 |
0 |
__+1__ |
2. |
Did the adverse event appear after the suspected drug was administered? |
+2 |
-1 |
0 |
__+2__ |
3. |
Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? |
+1 |
0 |
0 |
__+1__ |
4. |
Did the adverse reactions appear when the drug was readministered? |
+2 |
-1 |
0 |
__0__ |
5. |
Are there alternative causes (other than the drug) that could on their own have caused the reaction? |
-1 |
+2 |
0 |
__0__ |
6. |
Did the reaction reappear when a placebo was given? |
-1 |
+1 |
0 |
__0__ |
7. |
Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? |
+1 |
0 |
0 |
__0__ |
8. |
Was the reaction more severe when the dose was increased or less severe when the dose was decreased? |
+1 |
0 |
0 |
__0__ |
9. |
Did the patient have a similar reaction to the same or similar drugs in any previous exposure? |
+1 |
0 |
0 |
__0__ |
10. |
Was the adverse event confirmed by any objective evidence? |
+1 |
0 |
0 |
__+1__ |
Total Score |
5 |
||||
Total Score ADR Probability Classification
□ 9 Highly Probable
■ 5-8 Probable
□ 1-4 Possible
□ 0 Doubtful
Leflunomide has been approved in Taiwan since 2002. Many physicians may have a few clinical experiences in using leflunomide. In other countries have been highlighted the safety of this drug monitoring. However, Taiwan is a highly prevalence area of hepatitis B and hepatitis C. This case report is mainly to remind healthy care providers in using leflunomide may cause abnormal liver function. Therefore we strongly recommend that physicians need to routinely monitor patient's liver function while on leflunomide for minimizing the potential ADR in the future.
Reference:
1. Siva C, Eisen SA. Leflunomide Use During the First 33 Months After Food and Drug Administration Approval: Experience With a National Cohort of 3,325 Patients. Arthritis & Rheumatism 2003; 49 (6): 745-751.
2. Li EK. Tam LS. Tomlinson B. Leflunomide in the treatment of rheumatoid arthritis. Clinical Therapeutics 2004; 26(4):447-459.
3. Collier G, Page FD. A case of acute pneumonitis associated with leflunomide treatment. Respiratory Medicine Extra 2005; 1(3):35-37.
4. Sevilla-Mantilla C. Ortega L. Agundez JA. Leflunomide-induced acute hepatitis. Digestive & Liver Disease 2004; 36(1):82-84.
5. Cannon Gw, Kremer JM. Leflunomide. Rheum Dis Clin N Am 2004; 30(2):295-309.
6. EMEA public statement on leflunomide (Arava): severe and serious hepatic reactions [doc ref EMEA/5611/01/en]. London (UK): European Agency for the Evaluation of Medicinal Products. 2001/03/12. Available: www.emea.eu.int/htms/human/drugalert/drugalert.htm.
7. van Roon EN. Jansen TL. Houtman NM. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: incidence and severity of hepatotoxicity. Drug Safety 2004; 27(5):345-352.
8. Naranjo CA, Busto U, Sellers EM. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-245.
9. Kaplowitz N. Drug-induced liver injury. Clin Infect Dis 2004; 38(Suppl 2): S44.
10. Shpaner A, Li W, Ankoma-Sey V, Botero RC. Drug-induced liver injury: hepatotoxicity of quetiapine revisited. Eur J Gastroenterol Hepatol. 2008; 20(11): 1106-1109.
摘要
Leflunomide是針對潛在病因進行改善疾病修飾的抗類風濕性關節炎藥品,服用後經由肝快速代謝成活性代謝物A771726,此代謝物因具有較長之半衰期,因此對於肝臟有潛在的蓄積毒性。本案例為五十三歲男性,因風濕性關節炎而使用leflunomide治療,於服藥後一個半月出現黃疸症狀以及肝功能指數異常,Naranjo scale評估為極可能(Probalbe)是leflunomide引起的急性肝衰竭。自leflunomide上市後,其在肝毒性之副作用已逐漸引起重視及警惕,希望藉此病例報告能提醒使用leflunomide時應注意監測病患肝功能的變化。
作者
義大醫院藥劑部藥師 劉慧萍、謝坤屏、蔡斌智