Abstract
Rituximab is a human/mouse chimeric anti-CD20 antibody, used as monotherapy or in combination with chemotherapy for B-cell non-Hodgkin's lymphoma. The most common adverse effects are infusion-related reactions (fever, chills, and urticaria) that usually occur within a few hours after infusion. Hematological adverse effects have been observed in patients receiving rituximab monotherapy, but delayed-onset neutropenia is relatively uncommon. Here we report a case of febrile delayed-onset grade 4 neutropenia associated with rituximab combination with CHOP chemotherapy (R-CHOP). A 32 year-old female, 29 days after receiving the 7th cycle of R-CHOP developed fever and chills, and febrile delayed-onset neutropenia was suspected. Antibiotic and G-CSF were used, the response to G-CSF diminished and the duration of G-CSF usage prolonged to meet the need for neutrophil recovery to normal level. After rule out other causes such as infections, the result of bone marrow examination, the Naranjo probability test revealed a probable correlation, and no other medications used during the episode of neutropenia, the episode was highly suspected to be rituximab-related in the R-CHOP regimen.
Although rituximab-induced delayed-onset neutropenia is uncommon, it should still be kept in mind. Accordingly, the blood cell count should be monitored for a longer duration. Although G-CSF is not recommended by the ASCO guideline, in our case, G-CSF seems helpful, we thought it would be better to administer it for prophylaxis rather than for treatment.
Key words: rituximab, delayed-onset neutropenia, G-CSF
Introduction
Rituximab is a human/mouse chimeric anti-CD20 antibody, which is indicated for the treatment of patients with CD20-positive B-cell non-Hodgkin's lymphoma (NHL). Rituxmab is used as monotherapy or in combination with CVP (cyclophosphamide, vincristine, prednisolone) chemotherapy for low-grade or follicular NHL, and in combination with CHOP (cyclophosphamide, low-dose doxorubicin, vincristine, prednisolone) chemotherapy for diffuse large B-cell NHL. The most common adverse effects are infusion-related reactions (fever, chills, and urticaria) that usually occurred within a few hours of starting the first infusion. In clinical trials, grade 3 and 4 hematological adverse effects including neutropenia (4.2%), leukopenia (2.8%), and thrombocytopenia (1.7%) had been observed in patients receiving rituximab monotherapy. During post-marketing surveillance from manufacturer, rare delayed-onset neutropenia (0.02% rate) had also been noticed, which occurred more than 4 weeks after the last infusion of rituximab. Thereafter, more and more cases of delayed-onset neutropenia of rituximab use were reported1-4. Recently, a study in Japan suggested that the incidence of delayed-onset grade 3 and 4 neutropenia was as high as 20.4% after rituximab-containing chemotherapy5. In Taiwan, no similar study published, here we report a case of febrile delayed-onset grade 4 neutropenia associated with rituximab combination with CHOP chemotherapy.
Case Report
A 32 year-old female was diagnosed to have diffuse large B cell lymphoma according to the World Health Organization classification of lymphoid malignancies and CD20-positive lymphoma was confirmed. Eight cycles of standard CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) every 3 weeks plus rituximab regimen (R-CHOP) were scheduled. Each cycle consisted of intravenous injection of rituximab 375 mg/m2 (600 mg) on day 1, on day 3, cyclophosphamide 750 mg/m2 (1200 mg), doxorubicin 50 mg/m2 (80 mg), and vincristine 1.4 mg/m2 (2 mg) were added. Prednisolone 100 mg was also given orally per day from day 3 to day 7. Since the third cycle of R-CHOP, febrile grade 4 neutropenia occurred and she was then given one dose of granulocyte-colony stimulating factor (G-CSF) 300 mcg subcutaneously. In the following four cycles, more doses of G-CSF per cycle were needed to reach the same response, hence the dosage of G-CSF was escalatory to 3 or 6 doses per cycle.
Twenty-nine days after the seventh cycle, the patient presented with fever and chills. No other prescription medications were used within that period. Physical examination showed body temperature of 38.9℃, blood pressure 110/70 mmHg, heart rate 100 beats/min, and respiration rate 18 breaths/min. Laboratory tests revealed white blood cell count 2.19 x 103/mm3 (reference range 4.0-10.0 x 103/mm3), absolute neutrophil count (ANC) 44/mm3, red blood cell count 3.86 x 106/mm3 (reference range 4.2-5.4 x 106/mm3), platelet count 335 x 103/mm3 (reference range 130-400 x 103/mm3), and the level of C-reactive protein was 4.46 mg/L (reference range 0-1 mg/L). As a febrile grade 4 neutropenia was diagnosed, she was then hospitalized and the eighth cycle of chemotherapy was not given.
Empirical antibiotic therapy with intravenous cefepime 2g every 12-hour intervals for 7 days was initiated. Bone marrow aspiration showed myeloid hypoplasia. Blood and bone marrow cultures yielded no growth of aerobic pathogens. Both cytomegalovirus (CMV) and Epstein-Barr virus (EBV) titers were negative for active infection. The patient had persistent fever with maximum temperature of 39.4℃ throughout the first 3 days. Although no specific pathogen was identified, the antibiotic regimen was shifted to a combination therapy, adding intravenous clindamycin 600mg every 8 h for 7 days starting from hospital day 3. During hospitalization, she was treated with G-CSF 300 mcg s.c. daily for 3 days, and neutropenia resolved within 4 days. The medications used during hospitalization, physical findings, results of laboratory tests and the responses of neutrophil to G-CSF are listed in Table 1- 3 and Figure 1.
Table 1 Medications used during hospitalization
Medications and dosage\date |
1/12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
Cefepime i.v. 2 g q12h |
||||||||||
Clindamycin i.v. 600 mg q8h |
||||||||||
G-CSF s.c. 300 mcg qd |
||||||||||
Acetaminophen 500 mg 1# prn |
x 1 |
x 4 |
x 3 |
qd |
qd |
qd |
qd |
qd |
||
Metoclopramide 5 mg st |
||||||||||
Sennoside 2# hs |
Table 2 Cultures during hospitalization
Items\Date\results |
1/12 |
1/12 |
1/18 |
Specimens |
Blood |
Bone Marrow |
|
Cultures |
Negative |
Negative |
|
Sensitivity tests |
No aerobic pathogen |
No aerobic pathogen |
|
CMV IgG Ab |
(+) 165.9 |
||
CMV IgM Ab |
(-) 0.15 |
||
E-B VCA IgM Ab |
(-) 0.02 |
||
UGI panendoscope |
Negative |
||
Colonoscopy |
Colon polyps |
Table 3 Laboratory tests during hospitalization
Items |
Normal levels\dates |
1/12 |
1/13 |
1/14 |
1/15 |
1/16 |
1/18 |
1/20 |
WBC |
4.0-10.0 *103/cell/mm3 |
2.19 |
3.12 |
5.05 |
6.87 |
20.70 |
19.23 |
13.04 |
Seg |
40-74 % |
2 |
6 |
6 |
24 |
45 |
53 |
53 |
Band |
0-5 % |
0 |
0 |
2 |
4 |
13 |
12 |
7 |
ANC |
44 |
187 |
404 |
1924 |
12006 |
12500 |
7824 |
|
Lympho S |
19-48 % |
32 |
19 |
40 |
21 |
13 |
8 |
8 |
Mono |
3.4-11 % |
64 |
75 |
52 |
44 |
15 |
2 |
13 |
Eos |
0-7 % |
0 |
0 |
0 |
5 |
1 |
1 |
1 |
Baso |
0-1.7 % |
2 |
0 |
0 |
0 |
1 |
1 |
0 |
RBC |
4.2-5.4 *106/cell/mm3 |
3.86 |
4.03 |
3.76 |
3.76 |
3.71 |
3.88 |
3.72 |
Hb |
12-16 g/dL |
11.8 |
11.8 |
11 |
10.7 |
10.7 |
11.2 |
10.7 |
Ht |
37-47 % |
35.3 |
36.6 |
33.6 |
33.9 |
33.2 |
35.1 |
33.5 |
MCV |
81-99 fL |
91.5 |
90.8 |
89.4 |
90.2 |
89.5 |
90.5 |
90.1 |
MCH |
26-34 Pg |
30.6 |
29.3 |
29.3 |
28.5 |
28.8 |
28.9 |
28.8 |
MCHC |
33-37 g/dL |
33.4 |
32.2 |
33.7 |
31.6 |
32.2 |
31.9 |
31.9 |
PLT |
130-400 *103/cell/mm3 |
335 |
333 |
334 |
368 |
392 |
376 |
344 |
Promyelocyte |
1.9-4.7 % |
9.8 |
2 |
3 |
3 |
|||
Myelocyte |
8.5-16.9 % |
6 |
2 |
17 |
15 |
|||
Metamyelocyte |
7.1-24.7 % |
2 |
8 |
3 |
||||
NRBC |
1 |
|||||||
BUN |
8-25 mg/dL |
11 |
10 |
|||||
Scr |
0.5-1.5 mg/dL |
0.8 |
0.5 |
|||||
GOT/GPT |
5-35 IU/L |
17/13 |
37/17 |
|||||
LDH |
95-215 IU/L |
273 |
||||||
Na+ |
134-149 mEq/dL |
136 |
135 |
|||||
K + |
3.5-5.2 mEq/dL |
4.1 |
4.5 |
|||||
CRP |
0-1 mg/L |
4.463 |
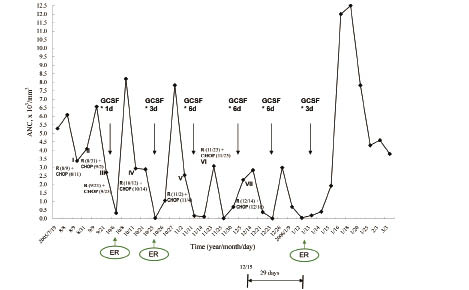
Fig. 1 Responses of neutrophils to G-CSF injections.
Discussion
Rituximab combined with CHOP chemotherapy is the standard regimen that serves as the first-line treatment for diffuse B-cell lymphoma. The results of clinical trials suggest that rituximab might increase the rate of complete response, prolong event-free and overall survival, and reduce the risk of treatment failure and death to CHOP regimen without a clinically significant increase in toxicity6-8. According to the manufacturer, the incidence of grade 3 and 4 neutropenia was slightly higher in the R-CHOP group (97%) than in the CHOP group (88%)8, but no difference between the two-treatment groups was noted in grade 2 to 4 infections (55.4% for R-CHOP vs 51.5% for CHOP).
Delayed-onset of grade 4 neutropenia is a vague complication of treatment with rituximab-based chemotherapy, which is defined when the ANC is less than 0.5 x 103/mm3 [according to the National Center Institute (NCI) Common Toxicity Criteria] and the adverse effect that occurred more than 4 weeks after the last infusion of rituximab.
In previously published reports, delayed-onset grade 4 neutropenia caused by rituximab alone or rituximab chemotherapy usually occurred within 28 to 175 days after administration and the nadir neutrophil count usually ranged from 0.02 x 103/mm3 to 0.39 x 103/mm3 1,4,9. A study of 53 patients showed that there was a high incidence of delayed-onset grade 4 neutropenia (15%) observed within 1 and 5 months, the characteristics of neutropenic episode included fever, infections (buccal cellulites, pneumonia, upper respiratory infection), and some may even occur asymptomatically1.
The mechanism for developing delayed-onset grade 4 neutropenia after rituximab remains unknown, but previous studies suggested it may be immune-mediated1,4,9. It is unlikely that rituximab causes severe neutropenia directly, due to the absence of CD20-coated neutrophils and hematopoietic cells8. Possible mechanisms include production of antineutrophil antibodies, apoptosis of mature neutrophils by the secretion of Fas (a molecule expressed on a variety of cells, which acts as a target for ligation by FasL on the surface of cytotoxic lymphocytes) related to hyperproliferation of large granular T cells, and immune dysfunction1,9. However Fukuno et al failed to identify antineutrophil antibody or immune complex during 3 episodes after rituximab therapy4. Additionally, the perturbation of stromal derived factor-1 (SDF-1) is associated with rapid B cell recovery and retardant neutrophil egress from the bone marrow following rituximab-basd therapy10. The authors demonstrated that delayed-onset neutropenia is self-limited and does not cause significant clinical events4,10.
In our patient, throughout the third and subsequent cycles of chemotherapy, episodes of grade 4 neutropenia with or without fever occurred about 1 week after treatment with R-CHOP regimen, and administration of G-CSF seemed to be helpful for ANC recovery to normal level. But more doses of G-CSF were needed for the later cycles. During the seventh cycle of R-CHOP chemotherapy, although 6 daily consecutive doses of G-CSF were given, the ANC did not recover as anticipated, but further decreased dramatically and grade 4 neutropenia on day 29 was found.
Use of the Naranjo probability scale indicated a probable relationship between R-CHOP and delayed-onset grade 4 neutropenia in our patient11. In addition, the bone marrow aspiration in our patient showed disturbance of myeloid maturation rather than myelosuppression for cytotoxic agents. These findings indicate that rituximab likely induce delayed-onset grade 4 neutropenia.
The necessity of G-CSF supported in literature reviews of delayed-onset grade 4 neutropenia remains controversial. The American Society of Clinical Oncology (ASCO) guidelines for the use of hematopoietic colony-stimulating factors (CSFs) recommend that CSFs should not be routinely used as adjuvant therapy for the treatment of uncomplicated fever and neutropenia12. However, other reports implied that delayed-onset febrile neutropenia have been resolved by using G-CSF when the ANC nadir was less than 0.06 x 103/mm3 1,9. It has been reported that the subsequent cycles of R-CHOP chemotherapy could be treated with G-CSF as prophylaxis for subsequent cycles of chemotherapy after an episode of febrile neutropenia7. Our patient was treated with G-CSF when the neutropenia developed again. Consequently the responses of ANC were not acceptable until the dose of G-CSF was escalated. A study suggested that a decrease of the dose of cyclophosphamide and doxorubicin by 50 percent should be considered when grade 4 neutropenia persisted during the next cycle. Nevertheless, the ANC was less than 1.5 x 103/mm3 before a scheduled cycle, the cycle might be delayed for up to 2 weeks7. Furthermore, the neutrophil count should be monitored over an 8-week period following rituximab therapy.
A randomized phase II study recognized that although major hematological toxicity was neutropenia and grade 4 neutropenia was observed in 85% of patients receiving standard R-CHOP chemotherapy, these hematological adverse effects were manageable with or without using G-CSF13.
In our patient, since no other medications were used during the episode of neutropenia, and no other diseases were attributable to the cause of neutropenia, we highly suspect that the episode was riuximab-related in the R-CHOP regimen.
Conclusions: Although rituximab-induced delayed-onset neutropenia is uncommon, physicians should keep in mind and monitor the blood cell count for a longer duration, and although G-CSF was not recommended by the ASCO guideline, in our case, G-CSF seems helpful and it would be better that G-CSF can be administered for prophylaxis rather than for treatment.
References:
1. Chaiwatanatorn K, Lee N, Grigg A, et al: Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol 2003; 121: 913-918.
2. Motl SE, Baskin RC: Delayed-onset grade 4 neutropenia associated with rituximab therapy in a patient with lymphoma: case report and literature review. Pharmacotherapy 2005; 25: 1151-1155.
3. Larrar S, Guitton C, Willems M, et al: Severe hematological side effects following Rituximab therapy in children. Haematologica 2006; 91(8 suppl): S11-12.
4. Fukuno K, Tsurumi H, Ando N, et al: Late-onset neutropenia in patients treated with rituximab for non-Hodgkin's lymphoma. Int J Hematol 2006; 84: 242-247.
5. Nitta E, Izutsu K, Sato T, et al: A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: a single-institution study. Ann Oncol 2007; 18: 364-369.
6. Vose JM, Link BK, Grossbard ML, et al: Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. J Clin Oncol 2001; 19: 389-397.
7. Coiffier B, Lepage E, Briere J, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 235-242.
8. Kimby E: Tolerability and safety of rituximab (MabThera). Cancer Treat Rev 2005; 31: 456-473.
9. Voog E, Morschhauser F, Solal-Céligny P: Neutropenia in patients treated with rituximab. N Engl J Med 2003; 348: 2691-2694.
10. Dunleavy K, Hakim F, Kim HK, et al: B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood 2005; 106: 795-802.
11. Naranjo CA, Busto U, Sellers EM, et al: A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-245.
12. Ozer H, Armitage JO, Bennett CL, et al: American Society of Clinical Oncology. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 2000; 18: 3558-3585.
13. Ogura M, Morishima Y, Kagami Y, et al: Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma. Cancer Sci 2006; 97: 305-312.
摘要
Rituximab 是抗CD-20的單株抗體,可單一療法或合併化學療法B瀰漫性大細胞淋巴瘤。較常見的副作用多與輸注有關如發燒、寒顫及蕁麻疹等,這些反應通常在開始輸注後的幾小時內發生。單獨使用 Rituximab 有出現3至4級的血液學不良反應,但超過4週而發生延遲性嗜中性白血球減少症則較罕見 (指超過4週才發生的)。本例報告一例使用 Rituximab 合併 CHOP(R-CHOP)而出現 4級延遲性嗜中性白血球減少症。這是一位32歲女性,罹患B瀰漫性大細胞淋巴瘤,在接受 R-CHOP 第7個療程後29天,出現發燒、寒顫及4級嗜中性白血球減少,懷疑延遲性嗜中性白血球減少症而住院。住院期間給予經驗性抗生素和皮下注射 G-CSF。對 G-CSF 的反應有越來越差的現象,需連續施打較長的療程才能使白血球回復至正常值。在排除其他疾病的因素,如病毒及細菌的感染、骨髓檢查的結果、Naranjo 相關性的分析呈現極有可能、病人在這段期間沒有使用其他處方藥品,故推測此熱性延遲性嗜中性白血球減少的不良反應,與 Rituximab 可能性比較高。對於使用 R-CHOP 曾發生 febrile neutropenia (FN) 的病人,有專家認為應在往後療程之後例行性注射 G-CSF,不需等到病人再次發生 FN 後才投予。如仍持續發生4級 neutropenia,則其他的併用藥品如 cyclophosphamide 與 doxorubicin 的劑量應減半;若ANC< 1500 cell/mm3,該次療程最多可延遲2星期,再繼續治療。此外,使用 rituximab 後,仍需持續監測白血球達8週。
雖然此種不良反應的發生率較罕見,醫療人員仍應謹記在心,對病人的血球數值的監測期要延長;雖然美國臨床腫瘤學會 (ASCO) 的治療指引不建議常規性給予 G-CSF,但對本個案是有需要的,而且預防性給藥可能比等到發生後才投予更有效。
作者
國泰綜合醫院藥劑科藥師 盧宜玲、黃婉翠、高啟蘭、林惜燕

