Abstract
A 33-year-old female was given parenteral ritodrine for 4 months in an attempt to suppress premature uterine contractions and developed acute pulmonary edema at the 33.5 weeks' gestation. Ritodrine was discontinued and furosemide and mechanical ventilation with high positive end-expiratory pressure (PEEP) were administered after emergent cesarean section with general anaesthesia was proceeded. The symptoms were subsided within 8 hours and the patient was extubated 24 hours post-delivery. Acute pulmonary edema on chest X-ray was resolved in 4 days and the patient was discharged 1 week later. The predisposing factors for the development of acute pulmonary edema were including premature labor, long-term use of betamimetic (beta-sympathomimetic) agent, tachycardia and rapidly increased body weight in the third trimester. With tocolytic therapy, betamimetic agents exert both chronotropic and inotropic effects on the maternal cardiovascular system and probably exacerbate the hyperdynamic and hypervolemic state of normal pregnancy. If tocolysis with betamimetic infusion is required, screening and monitoring of patients are warranted.
Key words: acute pulmonary edema, betamimetic agent, premature labor, ritodrine, tocolytic therapy
Introduction
Ritodrine is selective β2-sympathomimetic (betamimetic) agent that is useful for inhibition of uterine contractility in the patient with premature labor. The side effects of betamimetic therapy include hyperglycemia, hypokalemia, sodium and water retention, tachycardia, myocardial ischemia, and pulmonary edema. Although pulmonary edema has been infrequently reported, it is a serious complication that may cause maternal death.1 The benefit of betamimetic therapy on birth weight and perinatal mortality remains controversial.2,3 Familiarity with the clinical features of pulmonary edema related to betamimetic therapy should increase the ability of health care professionals to manage this critical complication.
Case Report
A 33-year-old female (gravida 3, para 0, abortus 2) with twins pregnancy by in vitro fertilization (IVF) was admitted for premature uterine contractions at 19th weeks' gestation. This patient did not smoke and had no preexisting medical history of cardiac or pulmonary disease. After admission, parenteral ritodrine was infused at increasing doses of 100-150 mcg/min for inhibiting preterm contractions and 5% dextrose solution was used as vehicle for the tocolytic therapy. Four hours after admission, cervical incompetence was diagnosed. After vaginal delivery of fetal A (Apgar 0/0), cervical cerclage was arranged and tocolytic therapy with ritodrine infusion was resumed. During hospitalization, the dose of intravenous ritodrine was increased to 250 mcg/min in an attempt to suppress frequent uterine contractions. The infusion rate of intravenous fluids, dose of ritodrine, vital signs and laboratory data were summarized on Table 1.
Table 1. Medication, vital signs and laboratory data
Gestational age (week) |
19 |
20 |
22 |
24 |
26 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
Ritodrine 50 mg/amp (mcg/min) |
150 |
150 |
250 |
250 |
250 |
250 |
250 |
250 |
250 |
250 |
250 |
250 |
D5W 500 mL/bag (mL/hr) |
100 |
100 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
L-R 1000 mL/bag (mL/hr) |
- |
- |
80 |
80 |
80 |
80 |
80 |
80 |
80 |
60 |
60 |
60 |
Systolic BP (mmHg) |
128 |
129 |
131 |
136 |
121 |
123 |
120 |
122 |
136 |
138 |
145 |
150 |
Diastolic BP (mmHg) |
76 |
69 |
73 |
71 |
78 |
63 |
80 |
66 |
85 |
86 |
83 |
98 |
Heart rate (bpm) |
80 |
102 |
106 |
122 |
101 |
101 |
114 |
103 |
108 |
105 |
103 |
115 |
Respiratory rate (bpm) |
18 |
20 |
18 |
20 |
18 |
19 |
19 |
19 |
18 |
18 |
22 |
36 |
Body weight (KG) |
60 |
- |
- |
- |
- |
- |
63 |
65 |
67 |
68.5 |
69 |
64* |
Blood sugar (mg/dL) |
126 |
- |
- |
110 |
- |
- |
85 |
- |
- |
- |
90 |
76 |
Creatinine (0.44 - 1.03 mg/dL) |
0.33 |
- |
- |
0.25 |
- |
- |
0.28 |
- |
- |
- |
0.39 |
0.57 |
Sodium (134-148 mEq/L) |
140 |
- |
- |
139 |
- |
- |
139 |
- |
- |
- |
140 |
140 |
Potassium (3.6 - 5 mEq/L) |
3.4 |
- |
- |
4 |
- |
- |
4 |
- |
- |
- |
3.9 |
4.7 |
Procalcitonin (≤ 2 ng/dL) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0.43 |
Albumin (3.3 - 5.5 mg/dL) |
3.44 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
White count (3.5 - 11 *1000/uL) |
11.1 |
- |
- |
4.9 |
- |
- |
4.6 |
- |
- |
- |
4.6 |
19 |
Hemoglobin (12-16 g/dL) |
8.5 |
- |
- |
10.7 |
- |
- |
11.2 |
- |
- |
- |
11.2 |
15 |
Hematocrit (36-46 %) |
24.8 |
- |
- |
32.1 |
- |
- |
33.4 |
- |
- |
- |
31.9 |
44.4 |
Platelet (150 - 400*1000/uL) |
165 |
- |
- |
208 |
- |
- |
117 |
- |
- |
- |
117 |
141 |
* Body weight after cesarean section.
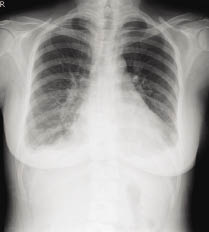
On the 102nd hospital day (at 33.5 weeks' gestation), however, the patient's breathing became labored and developed acute onset of dyspena. Thirty-five percent of oxygen therapy was supplied via face mask and the arterial blood gas values were pH 7.48, PO2 75.4 mmHg, and PCO2 27.4 mmHg with saturation 96.2%. The infusion rate of ritodrine was tapered to 200 mcg/min and intravenous fluid was held but in vain. On the next day, short of breath persisted still and the uterine contractions had become more and more intense. Emergent cesarean section without given steroid was performed due to fetal and maternal distress, and she delivered an Apgar 4/7, 2455 gm male infant under general anaesthesia. The chest radiograph (Fig. 1) showed bilateral perihilar infiltration and consolidation in bat-wing distribution consistent with acute pulmonary edema. After successful diuresis with IV furosemide and mechanical ventilation with high positive end-expiratory pressure (PEEP: 14 cmH2O), the patient's symptoms resolved within 8 hours and her endotracheal tube was extubated 24 hours post-delivery. Subsequent chest radiograph showed resolution of acute pulmonary edema in 4 days (Fig 2) and the patient was discharged 1 week later.
Figure 1 Chest radiograph showing bilateral alveolar infiltration without cardiac enlargement.
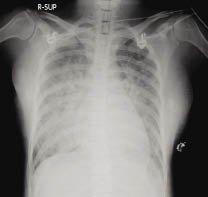
Figure 2 Resolution of acute pulmonary edema in 4 days after diuresis and mechanical ventilation with high PEEP.
Discussions
In normal pregnancy, the circulating blood volume increases 35-45% and ultimate result is a compensated high cardiac output condition. Labor may also produce profound changes, particularly when labor occurs before term.1,4 The most common causes of pulmonary edema in pregnancy are the use of tocolytic agents, underlying cardiac disease, fluid overload, and preeclampsia.5 Tocolytic agents induced pulmonary edema is a unique effect as a complication of betamimetic therapy for premature labor. The incidence varies in published references from 0.3% to 9.0%. This complication can be occurred during the therapy or up to 24 hours after discontinuation of the betamimetic agents.4
Typical manifestations include dyspnea, cough, chest pain and the physical examination reveals tachypnea, tachycardia, and auscultation reveals bibasilar crackles. Laboratory examination may reveal hypoxemia, hypokalemia, anemia and hypoalbuminemia. Roentgenograms of the chest usually show bilateral interstitial and alveolar infiltrates without cardiomegaly and pulmonary vascular redistribution.6,7 Diffrential diagnoses include pulmonary thromboembolism, amniotic fluid embolism, fluid overload, sepsis, hyperthyroidism, leukoagglutinin reaction, aspiration of gastric contents, pneumonia, severe preeclampsia and peripartum cardiomyopathy. 8
Risk factors of betamimetic agents related acute pulmonary edema include positive fluid balance, low levels of serum potassium, anemia, twin or multiple gestations, concomitant steroid use, general anaesthesia, sustained tachycardia greater than 140 beats per minute for prolonged periods, maternal infection and undiagnosed cardiopulmonary disease.1,4,6
The pathogenetic mechanism is still controversial. The betamimetic agents have been shown to stimulate the renin-aldosterone system and secretion of antidiuretic hormone and the result is a further expansion of circulating blood volume. Crystalloid infusion is typically given during tocolytic therapy to counteract hypotension caused by β2-adrenergic effect. The administration of saline rather than in 5% dextrose solutions has been shown to aggravate this increase in blood volume. In addition, salt and water retention may be potentiated by mineralocorticoid effects of antepartum glucocorticoid, which is given concomitantly to promote fetal lung maturity. Dilutional anemia and hypoalbuminemia result in decrease in intravascular colloid osmotic pressure and precipitate the development of hydrostatic pulmonary edema in patient with tocolytic therapy. Clinical studies suggest betamimetic agents also possess β1- mediated chronotropic and inotropic effects on the maternal cardiovascular system which may cause further changes in stroke volume, cardiac output and heart rate.1 Other factor, like general anaesthesia, is conceivable that even minimal myocardial depression could precipitate cardiac failure in the presence of hyperdynamic circulation secondary to pregnancy and betamimetic agents. Prolonged exposure to catecholamine which may cause myocardial dysfunction is another proposed mechanism. Increased capillary permeability from occult infection may also contribute to pulmonary edema. Circulating blood volume expansion is much greater in twin or multiple gestations and these patients is therefore theoretically more susceptible to further volume expansion in association with tocolytic infusion.9 In conclusion, if compensatory mechanisms fail or if high cardiac output stimulations are exaggerated, decompensation in the form of left ventricular failure and pulmonary edema may occur.
Management should consist of immediate discontinuation of the offending agent, administration of supplemental oxygen, and restriction of fluid intake along with intravenous loop diuretic therapy. If feasible, labor should be allowed to proceed. Patients who have severe respiratory failure may benefit from mechanical ventilation with positive end-expiratory pressure. Other possible causes of pulmonary edema, such as preeclampsia, heart failure and infection, should be excluded. Clinical improvement typically occurs within 12 to 24 hours. The risk of betamimetic agent-related pulmonary edema can be minimized by administering the lowest infusion rate with incremental dosing, and keeping the heart rate less than 120 beats per minute.6
For use in premature labor, the effective dose of parenteral ritodrine is usually between 150-350 mcg/minute.10 In our case, the dose of ritodrine infusion was moderate. The onset of acute pulmonary edema was infused ritodrine for 4 months and the incubation period may be as short as days to as long as 5 months after initiation of therapeutic course on literatures. The predisposing factors for development of acute pulmonary edema were premature labor, long-term use of ritodrine infusion, increased body weight rapidly in the third trimester (+ 2 KG/week) and increased heart rate to more than 100 beats per minute. This patient had no preexisting cardiac or pulmonary disease and the increasing body weight rapidly in the third trimester may be as a result of hyperdynamic and hypervolemic state of normal pregnancy and fluid overload caused by intravenous fluid infusion and betamimetic agent. The fluid balance and urine output were not recorded and echocardiogram was not performed for exclusion of heart disease. If tocolysis with betamimetic agents is obligatory, screening should include medical history and examination such as weight, serum electrolytes and glucose, full blood count and baseline ECG aimed at excluding the possibility of maternal cardiac disease. Monitoring during therapy must consist of measurement of fluid balance, pulse rate, blood pressure, respiratory rate and urine output and follow up of serum potassium, blood glucose and hematocrit periodically.
References:
1. Hawker F: Pulmonary oedema associated with β2-sympathomimetic treatment of premature labour. Anaesth Intensive Care. 1984; 12(2): 143-51.
2. The Canadian Preterm Labor Investigators Group. Treatment of preterm labour with the beta adrenergic agonist ritodrine. N Engl J Med. 1992; 327(5): 308-12.
3. Tan TC, Devendra K, Tan LK, et al: Tocolytic treatment for the management of preterm labour: a systemic review. Singapore Med J. 2006; 47(5): 361-6.
4. Bandi VD, Munnur U, Matthay MA: Acute lung injury and acute respiratory distress syndrome in pregnancy. Crit Care Clin. 2004; 20(4): 577-607.
5. Sciscione AC, Ivester T, Largoza M et al: Acute pulmonary edema in pregnancy. Obstet Gynecol. 2003; 101(3): 511-5.
6. Lee-Chiong T Jr, Matthay RA: Drug-induced pulmonary edema and acute repiratory distress syndrome. Clin Chest Med; 2004; 25(1): 95-104.
7. Ware LB, Matthay MA: Acute pulmonary edema. N Engl J Med. 2005; 353(26): 2788-96.
8. UpToDate: Pulmonary pearls: A 24 year-old woman with pulmonary edema after labor and delivery. Available at www.uptodate.com
9. Lamont RF: The pathophysiology of pulmonary oedema with the use of beta-agonists. BJOG. 2000; 107(4): 439-44.
10. Micromedex: Available at www.thomsonhc.com/micromedex2/librarian
摘要
本案例報告一位33歲女性於懷孕19週開始住院使用 ritodrine 注射劑安胎長達4個月,於33.5週孕程時突然發生急性肺水腫而緊急施行剖腹產順利產下胎兒,在施予氣管內管插管連接呼吸器給予氧氣治療及利尿劑使用後,症狀於8小時後緩解,1天後拔除氣管內管,病人於1週後出院。造成急性肺水腫可能之原因包括早產、長期使用 ritodrine、心跳過快、及懷孕後期體液滯留。
Ritodrine 造成之急性肺水腫其致病機轉為多重因素,正常懷孕過程即會造成心臟功能代償而提高心輸出量、血管內血液容積增加並伴隨膠體滲透壓的下降,安胎過程所使用之擬交感神經促進劑會使孕婦之心跳速度及心輸出量更進一步提升。以上多重因素交互作用之結果,可能會使得體液於肺部微血管分布失衡而造成急性肺水腫。
使用擬交感神經促進劑之前,應先排除病人是否具有潛在之心臟疾病,並且於用藥過程中規律監測病人之體液平衡、血壓、心跳速率、呼吸速度、及排尿量,並定期監測血中鈉離子、鉀離子、血糖、血紅素及血比容。
作者
林口長庚醫院藥劑部藥師 蕭旋玲、陳琦華、鄧新棠
林口長庚醫院胸腔科醫師 黃崇旂

