Abstract
Background/Aim: ESRD incidence in Taiwan ranked first and prevalence ranked second in the world from 2002 to 2005. Hemodialysis (HD) patients often require 12 medications to treat 5 to 6 comorbid conditions. HD patients use a large number of medications, which increases the risk for medication-related problems (MRPs). Thus, HD patients may be at particular risk for MRPs. Our aim is to analyze the efficacy of pharmaceutical care in MRPs in ambulatory hemodialysis patients.
Methods: This study is performed as a double-blind, randomized, active controlled trial. We invite and communicate with HD patients. After pharmaceutical evaluation, pharmacist do interventions to resolve MRPs in experimental group. In control group, pharmacists don't do pharmaceutical interventions. We follow each patient in two weeks and record the MRPs score.
Results: In pharmaceutical care group, at 2nd pharmaceutical visit semi-quantitative MRPs score was 0.79 ± 0.32 (Mean ± standard error of mean, S.E.M.). In control group, at 2nd pharmaceutical visit semi-quantitative MRPs score was 2.13 ± 0.49 (Mean ± S.E.M.)(P < 0.05). MRPs rate at 1st visit was 68.4% in pharmaceutical care group. After pharmaceutical care, MRPs rate at 2nd visit was 36.8% in pharmaceutical care group. In the other hand, MRPs rate at 1st visit was 73.9% in control group. Without pharmaceutical care, MRPs rate at 2nd visit remained at 65.2% in control group. Pharmaceutical care reduces MRPs rate in pharmaceutical care group.
Conclusion: Pharmacists solved MRPs significantly in pharmaceutical care group. Clinical pharmacists can reduce MRPs score and MRPs rate in ambulatory HD patients and provided positive effect in caring these patients.
Keywords: Efficacy, ambulatory hemodialysis, pharmaceutical care, medication-related problems
Introduction
End stage renal disease (ESRD) incidence in Taiwan ranked first and prevalence ranked second in the world from 2002 to 20051,2. This attracted great attentions in Taiwan society. In 1990, incidence of ESRD was 126 per one million people and in 2001, incidence of ESRD was 331 per one million people. In 1990, incidence of ESRD was 382 per one million people and in 2001, prevalence of ESRD was 1,322 per one million people in Taiwan. These new patients emerge from mid-age people, population of diabetes mellitus with nephropathy and elderly people3.
In elderly patients registered to receive home healthcare, 14% of hospital admissions were primarily caused by adverse drug reactions (ADRs). One-third of these ADRs were related to impaired renal function, generally in very old women4. In 1991, about 190,000 persons in the United States either underwent dialysis or received a renal transplant for end-stage renal disease. Hypertension was judged to be the underlying cause of the condition in 29% of these patients, second only to diabetes mellitus (36%)5. In addition, anti-hyperlipidemia medication use is quite common in hemodialysis patients such as statins or fibrates. Comorbid conditions such as hypertension, hyperlipidemia and diabetes mellitus contribute to a large number of drug use in hemodialysis patients.
It was reported that hemodialysis (HD) patients often require 12 medications to treat 5 to 6 comorbid conditions6. Besides, ESRD is a lifelong disease and medication compliance may diminish overtime. Thus, HD patients may be at particular risk for drug related problems. Our aim is to analyze the efficacy of pharmaceutical intervention in MRPs of ambulatory hemodialysis patients.
In one Japan study, pharmacists actively managed the erythropoietin therapy, and the therapeutic and pharmacoeconomic outcome was evaluated. Impact of a pharmacist-implemented anemia management in outpatients with end-stage renal disease in Japan presented positive effect. Active participation of pharmacists in management of renal anemia had great therapeutic and pharmacoecomic impact in Japan, as in North America7. In Taiwan, pharmacist is not an integral member of the dialysis health care team. Nevertheless, Taiwan pharmacists established computerized-assisted screening system for patient with renal insufficiency and improved the patient safety8. Pharmacists are trained to identify medication or drug-related problems, to resolve MRPs and improve patient adherence to medications. We wondered whether pharmacist in Taiwan can help in identifying and resolving MRPs in HD patients.
Purpose
We propose that pharmacist interventions would reduce semi- quantitative MRPs score in HD patients.
Methods
Pharmacist will invite and communicate with ambulatory HD patients in HD department of Tainan Sin-Lau hospital to identify MRPs. We have given subjects their informed consent and that the study protocol has been approved by the institute's committee on human research.
Our inclusion criteria were 20-96 years old HD patient taking medications prescribed by nephrologists with exclusion criteria: HD patients who refused informed consent, and those cognitive impaired and unable to talk or hearing disability.
In experimental group, pharmacist will do pharmaceutical interventions after pharmacist evaluation to resolve MRPs, drug-drug interactions etc. Before application of pharmaceutical recommendations, agreement of physician-in-charge was needed. In the active control group, pharmacist in this study will not do pharmaceutical interventions. Another pharmacist will follow included HD patient in two weeks and record the semi-quantitative MRPs score (primary outcome).Category of semi-quantitative MRPs score see table 1.
Table 1. Semi-quantitative MRPs score
Description of MRPs |
severity |
points |
Pharmacist should educate patients, or patient need drug-related laboratory monitoring. Additional medicine was not needed. However, patient did not know how to use medicine precisely, or patient had poor drug compliance or medicine-related laboratory data abnormality, need pharmacist's education, need to monitor medicine-related laboratory data |
Minor |
1 point |
Pharmacist should contact physician to modify pharmacotherapy (eg. resulted from medicine-related laboratory data abnormality) such as dosage, dosing frequency, or duration. |
Modest |
2 points |
Pharmacist should contact physician to modify pharmacotherapy (eg. resulted from medicine-related laboratory data abnormality) such as discontinue drugs, add drugs, or shift from one drug to another medicine. |
Major |
3 points |
Outcome measurement: Pharmacist A evaluated MRPs of this patient continuously in a 14-day time frame. On 15th day of study, chief of pharmacy assign another pharmacist (pharmacist B) to make second visit with study patient. Pharmacist B measured and recorded primary outcome and secondary outcomes. MRPs were recorded with the PCNE Classification V 6.29.
Primary outcome measures
Semi-quantitative MRPs score in each group (Time Frame: 14 days).
Clinical pharmacists provide pharmaceutical care in experimental group in order to reduce semi-quantitative MRPs score. With pharmaceutical care, we suppose that semi-quantitative MRPs score will less than the control group.
Sample size
The minimum sample size was calculated on the basis of a statistical significance (alpha error) of 5%, a power of 80%, corresponding to a beta error of 20%. The anticipated effect size is 1.0. The minimum total sample size sample size calculated (two-tailed hypothesis) is 34 subjects10.
Randomization
Patients were randomized to treatment groups using SNOSE11. Concealed envelopes are place in a box from lowest to next highest number to make sure that study randomization envelopes always be opened sequentially.
Statistical methods
We use independent sample t-test to compare the mean of MRPs score before pharmaceutical intervention (1st visit) and after pharmaceutical intervention (2nd visit, 14 days after recruitment) in experimental group.
Results
Trial profile please sees Figure 1.We randomly assigned 42 patients between May 2011 and September 2011. The baseline characteristics were similar between experimental and control group (Table 2).
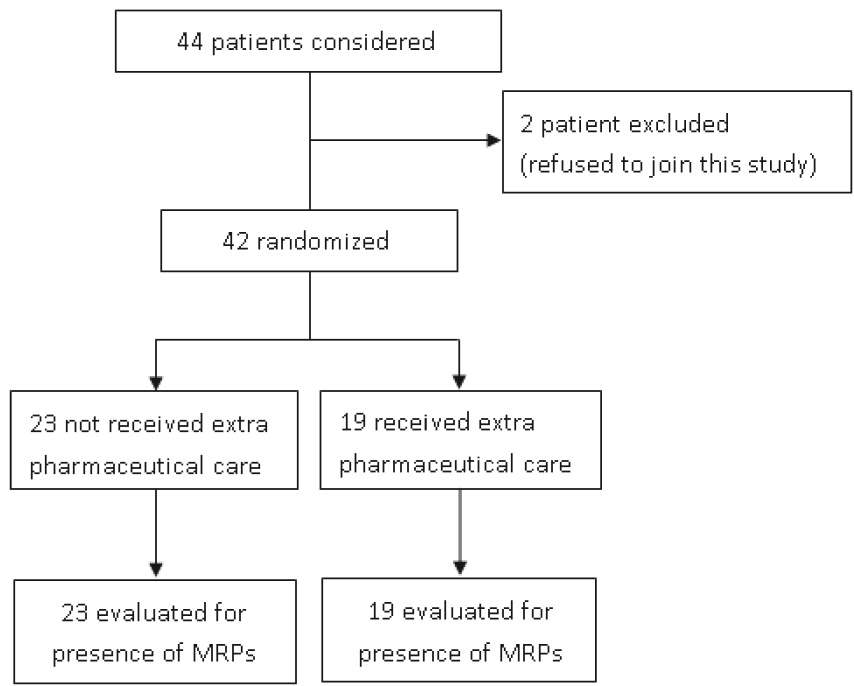
Figure 1. Forty-four patients were considered and two of them refused to join this study.
Table 2. Baseline Characteristics of the Patients.
Characteristic |
Pharmaceutical care (N=19) |
Control (N=23) |
P-value |
age |
57.3 ± 11.5 |
64.0 ± 12.8 |
0.086 |
Female sex (%) |
58 |
57 |
0.930 |
BMI (median) |
21.6 |
21.8 |
0.84 |
Comorbidities |
3.5 ± 1.4 |
3.4 ± 1.1 |
0.81 |
Drug used at 1st visit |
8.6 ± 4.4 |
9.2 ± 3.1 |
0.61 |
P-values were calculated with t-test.
In pharmaceutical care group, pharmacist found 1.11 ± 0.23 (mean ± standard error of mean, S.E.M) MRPs with MRPs score 1.95 ± 1.41 (mean ± S.E.M) points at 1st visit in average and 0.58 ± 0.21 (mean ± S.E.M) MRPs at 2nd pharmaceutical visit with MRPs score 0.79 ± 0.32 (mean ± S.E.M) in average (please see table 3 and figure 2). In control group, pharmacists found 1.26 ± 0.31 MRPs at 1st visit with MRPs score 2.35 ± 0.46 (mean ± S.E.M). At 2nd pharmaceutical visit in control group, pharmacists found 1.09 ± 0.24 MRPs with MRPs score 2.13 ± 0.49 (mean ± S.E.M). There was statistical significant between pharmaceutical care group and control group (P = 0.03, with independent sample t test) on MRPs score at 2nd pharmaceutical visit.
Table 3. MRPs score and amount of MRPs at first visit and second visit (SEM, standard error of mean). P-values were calculated with t-test.
|
Pharmaceutical care group mean ± SEM (N = 19) |
Control Group (N = 23) mean ± SEM |
P value |
|
First visit |
Amount of MRPs |
1.11 ± 0.23 |
1.26 ±0 .31 |
0.66 |
MRPs score |
1.95 ± 1.41 |
2.35 ± 0.46 |
0.53 |
|
Second visit |
Amount of MRPs |
0.58 ± 0.21
|
1.09 ± 0.24 |
0.12 |
MRPs score |
0.79 ± 0.32 |
2.13 ± 0.49 |
0.03 |
|
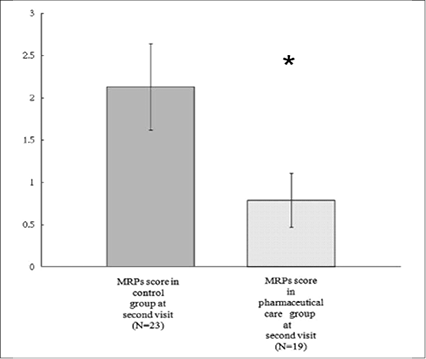
Figure 2. Mean of MRPs score (0.79 ± 0.32, Mean ± SEM) in pharmaceutical group and control group (2.13 ± 0.49) at second visit (SEM, standard error of mean ). *P<0.05
Incidence of MRPs was defined as percentage of patient with one or more MRPs among each group. Incidence of MRPs at 1st visit was 68.4% in pharmaceutical care group. After pharmaceutical care, incidence of MRPs at 2nd visit was 36.8% in pharmaceutical care group. In the other hand, incidence of MRPs at 1st visit was 73.9% in control group. Without pharmaceutical care, incidence of MRPs at 2nd visit remained 65.2% in control group (figure 3). Pharmacists did 11 pharmaceutical intervention and 10 of them were accepted.
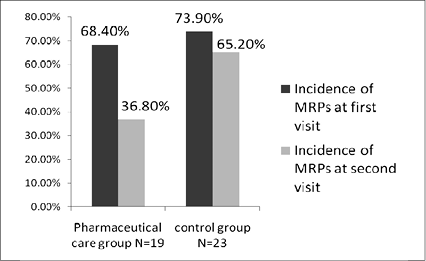
Figure 3. Incidence of MRPs at first pharmaceutical visit and second visit in each group.
Discussion
Several studies identify MRPs of HD patients over one or several months12-14. They did not show how important these identified MRPs were. Each MRP's importance seems the same in those studies; however, we do not think so. Our study was conducted to determine the reduction of semi-quantitative MRPs score which we think to be more precisely describe efficacy of contribution from pharmaceutical care.
We found 48 MRPs in our study, among them 21 MRPs were in experimental group (pharmaceutical care group, N = 19), and 27 MRPs were in the active control group (original health care team without pharmacists, N = 23).The causes of the MRPs might be related to doctors (64.58%), followed by patients (35.42%) among these 48 MRPs. The pharmacists solved 10 MRPs and reduce MRPs score from 37 points to 16 points in experimental group (N = 19). Our pharmaceutical interventions were based on spirit of evidence-based medicine; however, one pharmaceutical intervention was refuse by cardiologist, who thought HD patients could use hydrochlorothiazide while hydrochlorothiazide should be contraindicated in HD patients as listed in the package insert.
At second visits, pharmacists reduced MRPs score in pharmaceutical care group (0.79 ± 0.32) and reached statistical significance (P < 0.05) comparing to control group (2.13 ± 0.49) in a time frame of 14 days.
At first pharmaceutical visit, each pharmacist spent about one hour in face-to-face contact with patients gathering information and recording their current medication use and MRPs. In pharmaceutical intervention group, pharmacist needed extra half an hour to search information or article as evidence to resolve MRPs and communicate with physician or patient. At second visit, each pharmacist spent about half an hour in face-to-face contact with patients gathering information and MRPs. Incremental cost-effectiveness ratio (ICER) is commonly used when comparing two interventions (e.g., pharmaceutical intervention or not). The ICER is the difference between effectiveness (e.g., the reduction in the mean MRPs score in experimental group minus that reduction in mean in control group) divided by the difference between input costs between two groups. The input costs in this case may account for pharmacist time (in term of salary) mainly, any operation costs of pharmaceutical intervention and patient time for receiving the intervention. In pharmaceutical intervention group (N = 19), pharmacist did 10 pharmaceutical interventions in 38-hour pharmaceutical care and reduced the mean MRPs score from 1.95 to 0.79. In control group, reduction in mean MRPs score was 0.22 (2.35 minus 2.13). The average hourly wage of three pharmacists is about 260 NT$ (US$8.13). Difference between input costs between two groups was 1430 NT$ (US$ 44.71). ICER for reduction of each MRPs score cost about 1521 NT$ (US$ 47.56).
Conclusion
MRPs continue to occur at a high rate in ambulatory HD patients. Healthcare providers should be aware of this problem and efforts to avoid or resolve MRPs should be undertaken at all HD units. Clinical pharmacists in our hospital could play a positive role in the routine care of HD patients. Pharmacist should involve more in the care of MRPs of HD patients and pharmacists should be an integral member of the ambulatory hemodialysis health care team. We hope there will be more trials to help determining the efficacy (MRPs resolving ability or cost-effectiveness etc.) of pharmaceutical intervention. Our clinical trial was also a good material for further meta-analysis and clinical pharmacists could play a positive role in the routine care of ambulatory HD patients.
References:
1. United States Renal Data Systems (USRDS). 2002 Annual Data Report. National Institutes of Diabetes, Digestive and Kidney Diseases, Bethesda, MD.
2. United States Renal Data Systems (USRDS). 2005 Annual Data Report. National Institutes of Diabetes, Digestive and Kidney Diseases. Bethesda, MD.
3. Yang WC, Hwang SJ, and Taiwan Society of Nephrology. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant 2008; 23: 3977-82.
4. Helldén A, Bergman U, von Euler M, Hentschke M, Odar-Cederlöf I, Ohlén G. Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department: a retrospective study. Drugs Aging. 2009; 26(7):595-606.
5. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood Pressure and End-Stage Renal Disease in Men. N Eng J Med. 1996;334:13-18.
6. Manley HJ, Cannella CA, Bailie GR, St Peter WL. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis.2005; 46(4):669-80.
7. Kimura T, Arai M, Masuda H, Kawabata A. Impact of a pharmacist -implemented anemia management in outpatients with end-stage renal disease in Japan. Biol. Pharm. Bull.2004; 27(11):1831-33.
8. Ray CY, Niu SC, Yen SC, Chen WL, Chen HL, Deng ST. The efficacy of the computerized surveillance for patients with renal insufficiency. Form J Clin Pharm. 2008; 16(3):11-20.
9. Pharmaceutical Care Network Europe. PCNE Classification for drug-related problems V6.2. Available from: http://www.pcne.org/Documents/DRP/PCNE%20classification%20V6-2.pdf (14.01.2010)
10. Available from: http://www.danielsoper.com/statcalc3/calc.aspx?id=47
11. Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care 2005; (20):187-93.
12. Grabe DW, Low CL, Bailie GR, Eisele G. Evaluation of drug-related problems in an outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol. 1997;47(2):117-21.
13. Kaplan B, Mason NA, Shimp LA, Ascione FJ. Chronic hemodialysis patients. Part I: Characterization and drug-related problems. Ann Pharmacother 1994;28:316-19.
14. Kaplan B, Shimp LA, Mason NA, Ascione FJ: Chronic hemodialysis patients. Part II: reducing drug-related problems through application of the focused drug therapy review program. Ann Pharmacother 1994;28:320-24.
摘要
背景/目的:台灣末期腎病 (ESRD) 發病率曾在2002至2005年排名世界第一,而罹病率在世界上排名第二。血液透析 (HD) 病人往往有5-6種慢性病,需要12種藥物治療。血液透析病人使用了大量的藥物,這些藥物相關的問題的風險便增加。我們的目標是分析藥師照護對門診血液透析患者藥物相關問題分數降低的效果。
方法:這項研究是一項雙盲,隨機對照試驗。我們邀請血液透析病人加入研究,評估後,實驗組藥師介入做藥事照護,藉以解決藥物相關問題。在對照組,藥師不介入。兩個星期後,記錄每個病人藥物相關問題分數。
結果:藥師介入實驗組後,二次訪視藥物相關問題得分為0.79 ± 0.32,但對照組二次訪視得分高達2.13 ± 0.49 (平均值 ± SEM) (P < 0.05)。藥物相關問題盛行率在藥師第一次訪視,實驗組68.4%,藥師第二次訪視盛行率降至36.8%,對照組的藥物相關問題盛行率在藥師第一次訪視為73.9%,藥師第二次訪視時,盛行率保持在65.2%。
結論:臨床藥師介入可降低血液透析病人藥物相關問題分數,降低藥物相關問題盛行率,藥師對門診血液透析病人藥物相關問題之解決有正面的影響。
作者
台南新樓醫院藥劑科藥師 陳弘益、唐正乾、王悅琪、蔡佩瑜
台南新樓醫院腎臟內科醫師 黃麗雪

