Hyaluronidase for the Treatment of Mannitol Extravasation: A Case Report
Jah-Hsuan Wu, Yu-Ju Tseng
Department of Pharmacy, National Taiwan
University Hospital
Abstract
Hyaluronidase is a recombinant enzyme which is
indicated for absorption and dispersion of
injected drugs, subcutaneous fluid
administration and subcutaneous urography. The
use of hyaluronidase for hyperosmotic
extravasations constitutes a non-U.S. Food and
Drug Administration-approved use.
Mannitol is a hyperosmolar agent that reduces
intracranial pressure or cerebral edema.
Mannitol has an osmolarity of 1098 mOsm/L, and
its hyperosmolarity may cause severe edema or
swelling. We present a case in which
hyaluronidase was used to successfully treat
mannitol extravasation.
A 66-year-old man was admitted to hospital for a
scheduled brain tumor operation. The patient
underwent craniotomy for meningioma excision on
June 16, 2022. The patient received intravenous
infusions of 100 mL of 20% mannitol solution
every 8 hours for cerebral edema. Mannitol
extravasation with progressive edema was noted
at 16:00 on June 18, 2022. Symptoms included
right hand redness and severe swelling with
progressive edema, which influenced the bending
of the wrist. The patient then received 15 units
of hyaluronidase as 5 separate subcutaneous
injections along the leading edge of the
extravasation site at 19:30 and 21:30,
separately. Right hand redness, swelling, and
edema had nearly resolved the following day.
The exact dosage of hyaluronidase for
extravasation is not clearly defined and ranges
from 15–150 units. Our case report administered
cumulative dose of 30 units of hyaluronidase.
Hyaluronidase is an effective treatment for
mannitol extravasation.
Keywords: Hyaluronidase; Mannitol; Extravasation
1. Background
Hyaluronidase is a recombinant enzyme that
depolymerizes glycosaminoglycans such as
hyaluronic acid and chondroitin sulfate.
Hyaluronidase is believed to facilitate the
dispersion of injected agents by increasing
tissue permeability. The U.S. Food and Drug
Administration (FDA) has approved hyaluronidase
for the following indications: (1) subcutaneous
fluid administration (hypodermoclysis); (2) as
an adjuvant to accelerate the absorption and
dispersion of drugs in subcutaneous tissue or to
manage extravasation; and (3) as an adjunct to
promote the absorption of contrast media in
subcutaneous urography.1 The use of
hyaluronidase for hyperosmotic extravasations
constitutes a non-FDA-approved use.1
Mannitol is a hyperosmolar agent that reduces
intracranial pressure or cerebral edema.
Mannitol has an osmolarity of 1098 mOsm/L, and
its hyperosmolarity may cause severe edema or
swelling. The guidelines for the management of
mannitol extravasation include initiating
treatment with hyaluronidase, applying dry cold
compresses, and elevating the extremities. We
present a case in which hyaluronidase was used
to successfully treat mannitol extravasation.
2. Case Report
A 66-year-old man with a medical history of
hypertension, hyperlipidemia, benign prostatic
hyperplasia, and chronic periodontitis was
admitted to the hospital for a scheduled brain
tumor operation. After the detection of a
meningioma of the left anterior clinoid process,
the patient underwent craniotomy for meningioma
excision on June 16, 2022. The patient received
intravenous infusions of 100 mL of 20% mannitol
solution every 8 hours beginning on June 16,
2022, for cerebral edema. In addition, 100 mg
intravenous cefazolin sodium was administered
every 8 hours for postoperative prophylaxis.
Mannitol extravasation with progressive edema
was noted at 16:00 on June 18, 2022. Symptoms
included right hand redness and severe swelling
with progressive edema, which influenced the
bending of the wrist. Because the application of
a dry cold compress and the elevation of the
right wrist did not improve circulation, a
dermatologist and plastic surgeon suggested
administering a total of 15 units of
hyaluronidase as 5 separate subcutaneous
injections into the leading edge of the
extravasation site. The patient then received 15
units of hyaluronidase as 5 separate
subcutaneous injections along the leading edge
of the extravasation site at 19:30 and 21:30,
separately. The patient's pulse rate, capillary
refill time, color, and sensation were closely
monitored. Symptoms of extravasation such as
swelling improved 2 hours after hyaluronidase
administration. The patient's heart rate ranged
between 65 and 76 beats per minute. Right hand
redness, swelling, and edema had nearly resolved
the following day.
Dermatologic toxicity induced by mannitol
extravasation occurred. The patient had a
Naranjo score of 7 points, indicating a probable
adverse drug reaction (table 1). The patient's
renal and liver functions remained normal during
treatment. A serum creatinine of 1.1 mg/dL,
blood urea nitrogen of 14.8 mg/dL, and an
AST/ALT ratio of 22/23 U/L were reported. A
total of 30 units of hyaluronidase were
administered, and the extravasation symptoms
were successfully relieved.
Table 1 Naranjo score
3. Discussion and Conclusion
Hyaluronidase contains the polymers D-glucuronic
acid and D-N-acetylglucosamine, which are
composed of disaccharides linked by β-1,4 and
β-1,3 glycosidic bonds.1 It can break
down hyaluronic acid and glucosaminoglycans.
Hyaluronidase decreases the viscosity of
hyaluronic acid to improve the resorption rate
of fluid and tissue diffusion. Two published
case reports were reported of successful use of
hyaluronidase in mannitol extravasation. 2,
3
The exact dosage of hyaluronidase for
extravasation is not clearly defined.2,4,5
Previous studies especially for vinca
alkaloid extravasation have reported that a
higher dosage of 150 units/mL hyaluronidase
comprises 5 separate subcutaneous or intradermal
injections of 0.2 mL (30 units) along the
borders of the extravasation site using a 25- or
26-gauge needle. Administration of 1 ml
hyaluronidase solution for each ml of
extravasated vinca alkaloid-containing solution
and total dose 150-900 units of hyaluronidase
are recommended. 4-7 Alternatively,
clinicians may administer a lower dosage of
hyaluronidase with a diluted concentration of 15
units/mL according to the same dosing
instructions.2 Dosages of 15–25 units
of hyaluronidase are generally administered as 5
intradermal injections or through an injection
catheter along the borders of the extravasation
site.5
A higher dosage is recommended for patients
receiving treatment for chemotherapy
extravasations, particularly for those who have
undergone vinca alkaloid chemotherapy.7,8
Review articles have recommended higher dosages
for hyperosmolar agents, such as radiographic
contrast media, 10%–50% dextrose (504–2520 mOsm/L),
mannitol 20% (1098 mOsm/L), nafcillin (363 mOsm/L),
and phenytoin,4,5 whereas some case
reports have reported the administration of
lower dosages.2 In this case report,
a lower dosage of hyaluronidase was applied.
Hyaluronidase is typically reconstituted with
normal saline or 1% lidocaine to achieve 10–15
units/mL hyaluronidase.2
In this case report, 150 units of hyaluronidase
were mixed in 10 mL of normal saline, and 10
subcutaneous injections of 0.2 mL-hyaluronidase
were then administered along the leading edge of
the extravasation site. After giving 30 units of
hyaluronidase, swelling gradually diminished
after one hour. Redness, swelling, and edema had
nearly resolved by the following day. According
to the guideline, if extravasation occurs, nurse
should stop infusion first then local cooling or
warming compress for 15-20 minutes at least 4
times per day for 1-2 days, elevate extremity
and monitor extravasation site every 2-4 hours.9
Ideally administer hyaluronidase within 1 hour
after extravasation. Hyaluronidase was limited
to be prescribed by dermatology in our hospital
and it was one of reasons why we delayed
administrating hyaluronidase in this case. In
our case, doctor took 2 hours to ensure the
effects of dry cold compress and elevation of
the wrist. Because non-pharmacological treatment
was in vain, doctor consulted dermatologist for
hyaluronidase therapy. According to Reynolds et
al, 15 units hyaluronidase was given at 19:30
but no improvement was observed. Due to dosage
range was hyaluronidase 15–25 units, another 15
units hyaluronidase was administered after 2
hours and extravasation site significantly
improved within 1 hour.2,5 Proper
time to consider hyaluronidase to treat
extravasation was less than 1 hour. Late
treatment in our case was due to late
observation of extravasation, evaluation of
non-pharmacological treatment and limitation of
hyaluronidase prescription in our hospital.
Although we did not administer hyaluronidase
less than 1 hour after extravasation, we
reported a successful case that late
hyaluronidase administration can also
successfully treat mannitol extravasation.
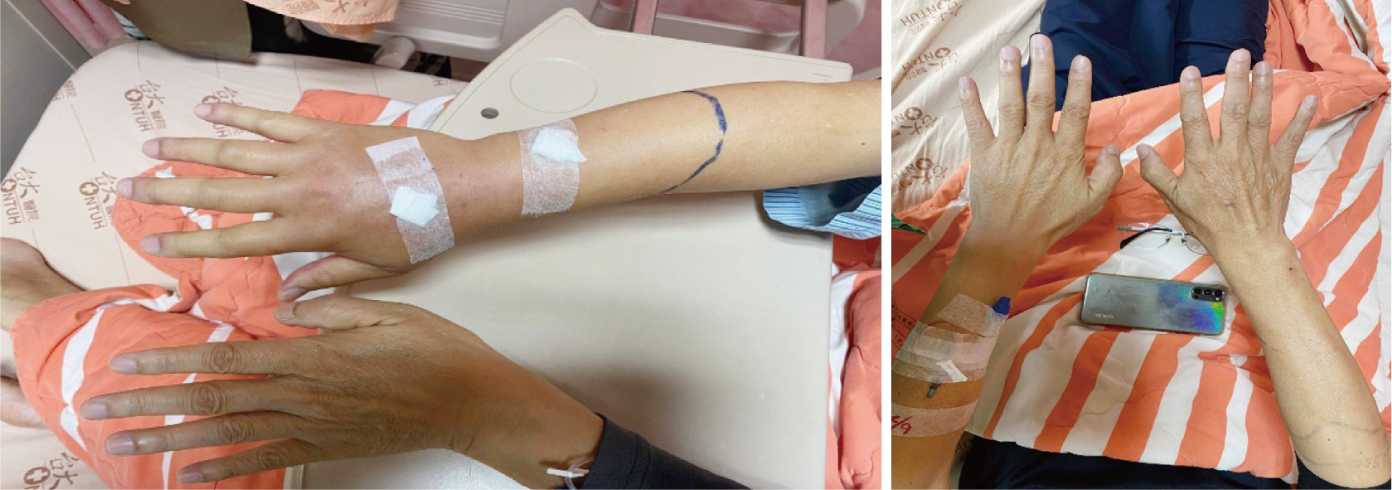
Figures 1 Progressive edema before treatment
(left). After treatment with hyaluronidase
(right).
Hyaluronidase should be self-paid for
extravasation management. It contains 1500 units
of hyaluronidase in 1 amp and 30 units of
hyaluronidase was used in this case. If the
patient did not response to hyaluronidase
therapy, he would receive fasciotomy. Although
it seems that hyaluronidase was not
cost-effective in this case, it prevented the
patient from fasciotomy and saved other surgical
fees.
According to Kaur1 Manbir et al, hyaluronidase
improves mild to moderate compartment syndrome.
It should be performed by certain protocol.3
When mannitol extravasation induce compartment
syndrome, we should evaluate the injury of
distal sensory and motor deficit and the
compartment pressure. If compartment pressure is
less than 30 mmHg and no distal sensory and
motor deficit, therapy of hyaluronidase 150
units in 10 ml normal saline by multiple
subcutaneous injections of 0.5-1 ml is
suggested. If compartment pressure more than 30
mmHg or severe compartment syndrome occurs,
immediate fasciotomy is first-line therapy.
Hyaluronidase can be used to treat extravasation
injuries from drugs such as mannitol, vinca
alkaloids, paclitaxel, phenytoin, 10%–50%
dextrose, total parenteral nutrition (TPN),
calcium salts (in the early stage), and
docetaxel.4 The early administration
of hyaluronidase in patients with extravasation
of hyperosmolar fluids such as mannitol, TPN,
10% dextrose, and 30% urea prevents the need for
fasciotomy.2 For reducing swelling
due to extravasation, the effects of
hyaluronidase are onset approximately 15–30
minutes.5 Certain patient
characteristics may influence the effect of
hyaluronidase. Older adults may be less
responsive to hyaluronidase due to their
inelastic skin; this treatment is more effective
in areas with a lower subcutaneous fat content.
Patients receiving large doses of
antihistamines, corticosteroids, salicylates, or
estrogens may also require higher doses of
hyaluronidase because such agents may cause
resistance to hyaluronidase.5 The
patient in this case report did not have any
characteristics that could diminish the effect
of hyaluronidase.
Mannitol extravasation was successfully treated
with 30 units of hyaluronidase.
Hyaluronidase is an effective therapy for mannitol extravasation to prevent surgical intervention.
玻尿酸分解酶於甘露醇外滲之案例報告
吳佳璇、曾郁茹
臺大醫院藥劑部
摘要
玻尿酸分解酶為一種合成酵素用於注射藥物的吸收和分佈、皮下注射輸液和皮下尿路造影。藥品仿單標示外也使用於高滲透藥物的外滲。
甘露醇藉由高滲透壓性質可用於降低腦內壓與減緩腦水腫,滲透壓為1098 mOsm/L,高滲透壓可能導致嚴重的水腫與腫脹。此篇描述一位成功藉由玻尿酸分解酶緩解甘露醇外滲的案例。
一位66歲的男性由於腦瘤安排住院開刀,2022年6月16日進行顱骨切開術,並且開始輸注每8時100毫升
20%甘露醇以治療腦水腫。自6月18日16:00發生甘露醇外滲與水腫,症狀包含右手紅腫與漸進式的腫脹,並且影響手腕的彎曲。病人接受玻尿酸分解酶治療,共15單位分五次皮下注射於外滲部位的邊緣,並於當天19:30與21:30各執行兩次,完成共30單位注射療程,右手紅腫與脹痛於隔天幾乎緩解。
玻尿酸分解酶使用於外滲的劑量為15至150單位,此案例使用累積劑量30單位玻尿酸分解酶,證實玻尿酸分解酶對於甘露醇的外滲為有效的治療藥物。
關鍵字: 玻尿酸分解酶、甘露醇、外滲
References:
1. Jung H. Hyaluronidase: An overview of its
properties, applications, and side effects. Arch
Plast Surg 2020; 47 : 297-300.
2. Kumar MM, Sprung J. The use of hyaluronidase
to treat mannitol extravasation. Anesth Analg
2003; 97 : 1199-200.
3. Kaur M, Balakrishnan N, Gosal JS, et al.
Efficacy of Hyaluronidase in the Mannitol
Extravasation Induced Compartment Syndrome-A
Case Report and Review of Literature. Turk J
Anaesthesiol Reanim 2021; 49 : 329-33.
4. Le A, Patel S. Extravasation of Noncytotoxic
Drugs: A Review of the Literature. Ann
Pharmacother 2014; 48 : 870-86.
5. Reynolds PM, MacLaren R, Mueller SW, et al.
Management of extravasation injuries: a focused
evaluation of noncytotoxic medications.
Pharmacotherapy 2014; 34 : 617-32.
6. Boulanger J, Ducharme A, Dufour A, et al.
Management of the extravasation of anti-neoplastic
agents. Support Care Cancer 2015; 23 : 1459-71.
7. Pérez Fidalgo JA, García Fabregat L,
Cervantes A, et al. Management of chemotherapy
extravasation: ESMO--EONS clinical practice
guidelines. Eur J Oncol Nurs 2012; 16 : 528-34.
8. Hanrahan K. Hyaluronidase for treatment of
intravenous extravasations: implementation of an
evidence-based guideline in a pediatric
population. J Spec Pediatr Nurs 2013; 18 :
253-62.
9. Martin SM. Extravasation management of
nonchemotherapeutic medications. J Infus Nurs
2013; 36 : 392-6.
通訊作者:吳佳璇/電子信箱:111104@ntuh.gov.tw


