ABSTRACT
The population pharmacokinetic parameters of phenytoin were estimated using routine therapeutic drug monitoring data from 192 epileptic patients. The 225 serum concentration values at steadystate after repetitive oral administration were analyzed using JavaPK® program and Bayesian feedback method. The maximal elimination rate (Vm) and the Michaelis-Menten constant (Km) were 6.94±1.03 mg/d/kg and 4.29±1.10 μg/mL in 67.2kg male, and 7.52±0.85 mg/d/kg and3.78±1.12 μg/mL in 55.1kg female respectively. In addition, the Vm for phenytoin appeared to be 8.23% increased and the Km appeared to be 15.68% decreased in patients receiving carbamazepin concurrently(p<0.05). The population pharmacokinetic parameters of phenytoin will be useful for designing dosage regimens in epileptic patients.
INTRODUCTION
Pharmacokinetics is the study of the relationship between the dose of a drug and the number in which its plasma concentrations change over time. A pharmacokinetic model provides a mathematical representation of this relationship and relates the independent variables of time and dose to the dependent variable, plasma concentration, using pharmacokinetic parameters such as clearance (CL) and volume of distribution (Vd).1
The population approach to the analysis of pharmacokinetic data also provides estimates of the average value of pharmacokinetic parameters in a study population and gives a measure of the variability of these parameters within that population.2
Phenytoin (DPH) is an effective anticonvulsant that has been used for treatment of epilepsy for many years. DPH is a drug with a narrow therapeutic range, displays zero-order kinetics in the therapeutic range and significant interpatient variability in pharmacokinetic parameters. Use the therapeutic drug monitoring is thus indispensable in optimizing dose.3 In the individualization of DPH dosage, it is important to use estimates of Vm and of Km that are representative of the population concerned knowledge of factors influencing Vm and Km in a population should allow for an appropriate dose to have been determined earlier in the course of therapy.4-5 Various nomograms and Bayesian regression programs are available for dosage computation for DPH exhibiting Michaelis-Menten elimination kinetics. However, the utility of dose-adjustment methods can be highly dependent on the pharmacokinetic mo-del used and on accuracy of the population pharmacokinetic parameters. 6-7
It has been shown that the system utilizing the Bayesian feedback method performs better than all methods previously reported in DPH dosage adjustment on an individual basis.7-8 The therapeutic range of PHT is narrow with concentrations of 10-20 μg/mL in adults.9-10 Achieving this range with standard dosage regimens is made more difficult by the presence of non-linearity of DPH pharmacokinetics and large inter-subject differences in kinetic parameters. Consequently, when a small change in DPH dosage may produce a disproportionately large change in steady-state serum concentration. Therefore, in the design of a DPH dosage regimen is very important to use values of pharmacokinetic parameters, which are represent-ative of the population.
METHODS
During 4 years in the past, we retrospective selected 192 adult outpatients (133 males and 59 females) in KCMH Taiwan who had reliable measured data for the steady-state serum level of DPH, while they were taking different daily doses. The study includes criteria were elder than 20 years old Taiwanese, out patients who administrated oral DPH capsule for epilepsy. All the adult patients were given DPH capsule (Dilantin®brand capsules, Pfizer Pharmaceutical Co., Ltd.). The concentration of DPH was determinate at least 30 days after any change in dosage. All blood samples were drawn at approximately 2-5 hours after administration of a dose. The DPH concentration was routinely measured by fluorescence polarization. The coefficient of variation of this assay was less than 10%.
The Michaelis-Menten model was used to describe the pharmacokinetics of DPH.
Rij= 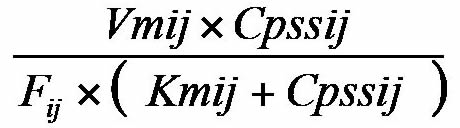
Where Rij is the daily dose of DPH for the ith Cpss in the jth patient (mg/day), Vmij is the ith maximum elimination rate (mg/day) for the jth patient, Kmij is the ith Michaelis-Menten constant (μg/mL) for the jth patient, Cpssij is the ith steady-state concentration (μg/mL) for the jth patient, and Fij is the bioavailability of DPH for the ith dosage from administration to the jth patient. Bioavailability is assumed to be 100%.
During the routine therapeutic drug monitoring of DPH, the serum DPH concentration was sometimes low in patients administered carbamazepine concurrently. Thus, the influence of carbamazepine on the Vm and Km of DPH was second view of this study when DPH co-administration of carbamazepine.
Data analysis was performed with the JavaPK®, which is a clinical pharmacokinetic (or therapeutic drug monitoring, TDM) tool for mobile devices. JavaPK® is the first clinical pharmacokinetic program designed for cell/mobile phones in the world, which is built with both Sawchuk-Zaske method and Bayesian estimation method. With Bayesian estimation, one can use any single-blood sample (at steady-state) to estimate individual pharmacokinetic parameters.
RESULTS
Routine clinical pharmacokinetic data collected from outpatients receiving DPH were analyzed to estimate population pharmacokinetic parameters. There were 225 steady-state DPH concentrations from 192 outpatients. The pertinent characteristics of the patient population show in Table I. Theses studies were taking DPH alone (102 patients) or DPH combined with other anticonvulsants (90 patients). Their ages and weights ranged from 20 years to 84 (mean 45.30 years, S.D. 17.0 years) and 28.5kg to 110.0 (mean 63.46 kg, S.D. 10.72kg). The mean daily dose of DPH was 312.76mg and the mean serum concentration was 12.03 μg/mL. The frequency distribution within the data set of their demographic factors: age, weight, daily dose and serum concentration are displayed in Fig. 1-4.
The results of our data analyses indicated that the maximal elimination rate (Vm) and the Michaelis-Menten constant (Km) were 6.94±1.03 mg/d/kg and 4.29±1.10 μg/mL in 67.2kg male, and 7.52±0.85 mg/d/kg and 3.78±1.12 μg/mL in 55.1kg female , average steady-state serum concentration were 12.24±7.57 in male and 11.55±7.76 in female respectively for patient population studied. The final population pharmacokinetic parameter shows in Table II.
The Vm and Km of a 63.46 kg adult outpatient taking DPH with carbamazepine were estimated to be 7.62±0.89 mg/d/kg and 3.55±1.35 μg/mL, respectively. (Table III ) The Vm for DPH appeared to be 8.23% increased and the Km appeared to be 15.68% decreased in patients receiving carbamazepin concurrently. (p<0.05)
CONCLUSION
The population pharmacokinetic parameters have an important role in current therapeutic monitoring. The Bayseium has been employed to obtain estimates of the population pharmacokinetic parameters from serum concentration measurements made in patients during routine treatment.
The conclusions of this analysis su-ggest that the Vm of DPH is higher in male than female, Km of DPH is lower in male than female. The second view of this study is slight but significant increase in serum concentration were observed in patients receiving cabamazepine currently. The Vm were decrease 22%, Km were increased 10% co-administrated carbamazepine. The population pharmacokinetics of phenytoin was studied using routine therapeutic drug monitoring data from 192 epileptic patients. The 225 serum concentration values at steady-state after repetitive oral administration. The population pharmacokinetic parameters of phenytoin will be useful for designing dosage regimens in Taiwan adult patients with epilepsy.
Table I. Description of patient data
Number of patient Male Female |
192 133 59 |
Age (years) |
45.3±17.00 |
Weight (kg) Total |
63.46±10.72 |
Weight (kg)Male |
67.17±9.37 |
Weight (kg)Female |
55.11±8.72 |
Daily dose of DPH (mg) |
312.76±66.56 |
Steady-state concentration, Css (µg/mL) |
12.03±7.62 |
Monotherpy |
102 |
Polytherapy |
90 |
Table II. Population pharmacokinetic parameters of DPH
Total n=192 |
Male n=133 |
Female n=59 |
P value |
|
BW |
63.46±10.72 |
67.17±9.37 |
55.11±9.72 |
0.000 |
Vm |
7.12±1.01 |
6.94±1.03 |
7.52±0.85 |
0.000 |
Km |
4.13±1.13 |
4.29±1.10 |
3.78±1.12 |
0.004 |
Css |
12.03±7.62 |
12.24±7.57 |
11.55±7.76 |
0.564 |
Table III. Population Pharmacokinetic Parameters of phenytoin Co-administered with Carbamazepine(CBZ)
Total |
Male n=133 |
Female n=59 |
|||||||
Co-administered with CBZ |
yes |
no |
P value |
yes |
no |
P value |
yes |
no |
P value |
Vm |
7.62±0.89 |
7.04±1.01 |
0.008 |
7.32±0.79 |
6.89±1.05 |
0.121 |
8.12±0.85 |
7.41±0.81 |
0.019 |
Km |
3.55±1.35 |
4.21±1.07 |
0.007 |
3.86±1.27 |
4.34±1.08 |
0.109 |
3.04±1.40 |
3.92±1.02 |
0.029 |
Css |
11.10±9.07 |
12.16±7.41 |
0.525 |
13.97±10.11 |
12.02±7.22 |
0.480 |
6.32±4.05 |
12.59±7.92 |
0.027 |
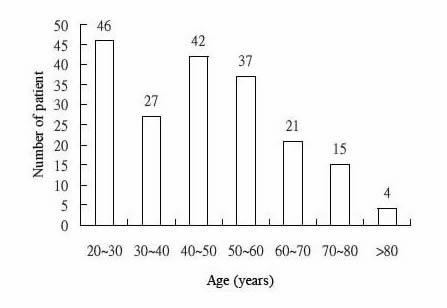
Fig.1. Distribution of ages
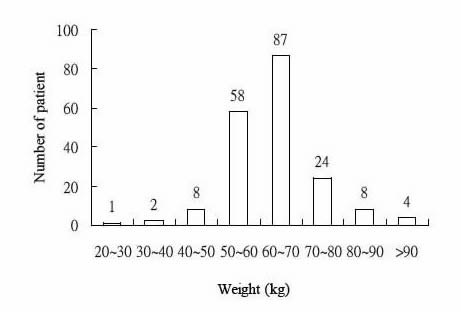
Fig.2. Distribution of weight
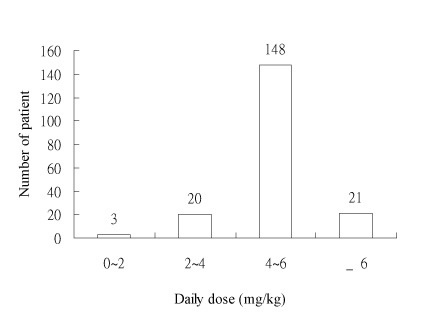
Fig.3. Distribution of daily dose
Fig.4. Distribution of serum concentration
ACKNOWLEDGMENT
The authors acknowledge Haw-Chyuan Lee, Shou-Yue Huang for their assistance with statistical analyses.
REFERENCES
1. Kimberley A.,Jackson and Sara E. Rosenbaum. The application of population pharmacokinetics to the drug development process. Drug Development and Industrial Pharmacy 24(2). 1155-1162,1998.
2. B. Whiting B, A. W. Kelman, and J. Grevel, population pharmacokinetics: theory and clinical application. Clin pharmacokinet.11,387-401(1986) -
3. Winter ME, Tozer TN. phenytoin. In: Evans WE, Schentag JJ, Jusko WJ, eds. Applied pharmacokinetics. Principles of therapeutic drug monitoring. Spokane. WA: Applied Therapeutics, 1987: 493-539.
4. Sheiner LB, beal SL. Bayesian individualization of pharmacokinetics: Simple implementation and comparison with non-Bayesian methods. J Pharm Sci 1982; 7112;1344-8.
5. Vozeh S, Miur KT, Sheiner LB, et al. Predicting individual phenytoin dosage. J Pharmacokinet Biopharm 1981; 92:131-46.
6. Hashimoto Y., Koue T., Otsuki Y., Yasuhara M., Hori R. and Inui K., J. Pharmacokinet. Biopharm., 8, 553 (1980).
7. Vozeh S. and Steimer J. L., Clin. Pharmacokinet., 10, 457 (1985).
8. Yukawa S., Higuchi K., Ohtsubo K. and Aoyama T., J. Clin. Pharm. Ther., 13, 293 (1988).
9. Lund L., Arch. Neurol., 31, 289 (1974).
10. Kutt H. and McDowall F., J. Am. Med. Assoc., 203, 969 (1968)
作者
高雄長庚紀念醫院藥劑科藥師 項怡平、溫軒琳、李炳鈺
高雄長庚紀念醫院神經科醫師 莊曜聰
1. Yi-Ping Hshinga, Yao-Chung Chuangb, Hsuan-Lin Wena and Ping-Yu Leea*
a Department of Pharmacy , Chang Gung Memorial Hospital at Kaohsiung, Kaohsiung County, Taiwan, R.O.C.
b Department of Neurology, Chang Gung Memorial Hospital at Kaohsiung, Kaohsiung County, Taiwan, R.O.C.

